Stable capping blocks calcium from binding to tissue*2
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Calcification is a leading cause of structural valve deterioration (SVD) in aortic tissue valves.1 RESILIA tissue effectively addresses calcification with advanced calcium-blocking.*2
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
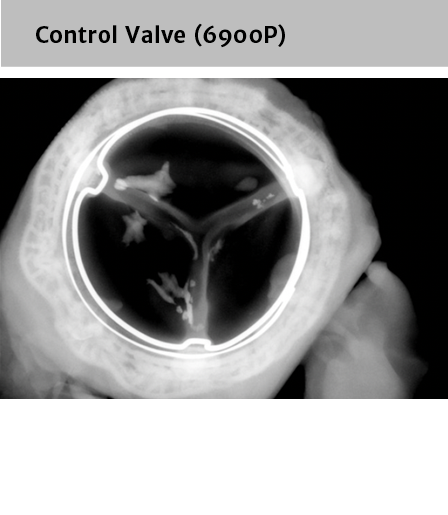
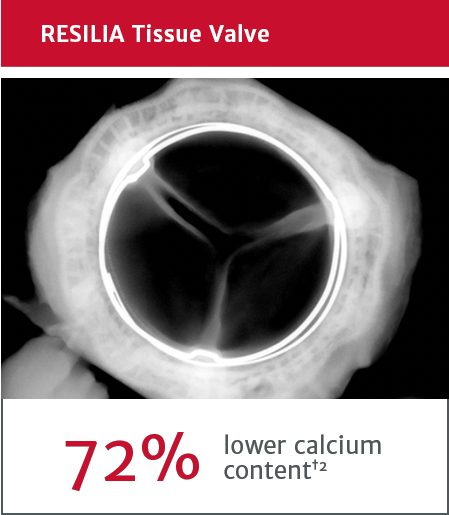
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
†RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg 2015;149:340-5.
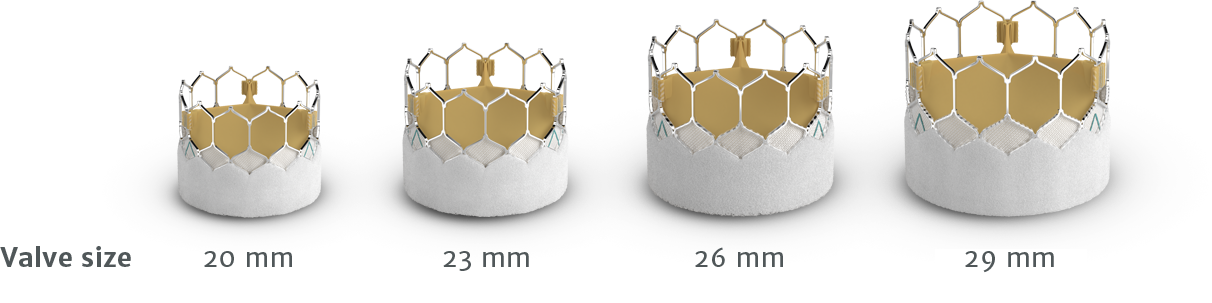
Scroll / swipe side to side to review valve line-up
*Compared to SAPIEN 3 valve
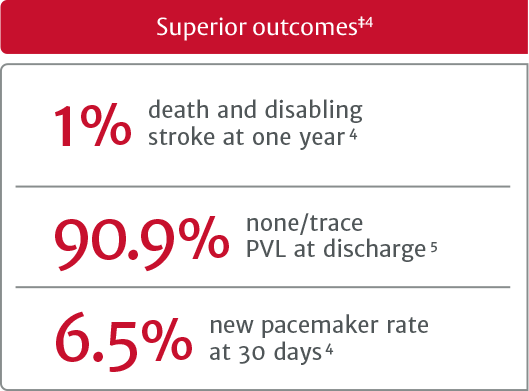
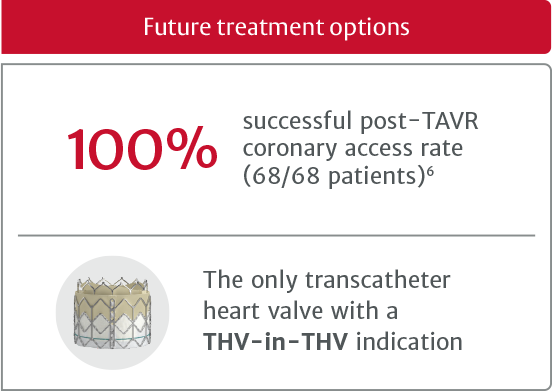
†In the PARTNER 3 trial, SAPIEN 3 TAVR was proven superior to surgery on the primary endpoint of all-cause death, all stroke, and reshospitilization (valve-related or procedure-related, and including due to heart failure) at one year and multiple prespecified secondary endpoints in low risk patients.
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
*SVD was adjudicated per Akins et al 2008.

Be the first to receive the latest news in transcatheter heart valves, including event announcements.
References: 1. Schoen F, et al. Calcification of Tissue Heart Valve Substitutes: Progress Towards Understanding and Prevention. Ann Thorac Surgery 2005 79;1072-80 2. Flameng W, Hermans H, Verbeken E, et al: Randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149(1):340-345. 3. Beaver T, Bavaria JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. Presented at the 103rd Annual Meeting of the American Association for Thoracic Surgery, May 2023. 4. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. NEJM. 2019; 380:1695-1705. doi:10.1056/NEJMoa1814052. 5. Nazif TM, Cahill TJ, Daniels D, et al. Real-world experience with the SAPIEN 3 Ultra transcatheter heart valve: a propensity-matched analysis from the United States. Circ Cardiovasc Interv. 2021;14:e010543. doi:10.1161/ CIRCINTERVENTIONS.121.010543. 6. Tarantini G, Nai Fovino L, Le Prince P, et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon-expandable valve. Circ Cardiovasc Interv. 2020;13:e008972. doi:10.1161/CIRCINTERVENTIONS.120.008972.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).