Controlling for the Future
Achieving higher standards for what today's patients will need tomorrow.
Controlling for the Future
Achieving higher standards for what today's patients will need tomorrow.
Thinking about the lifetime management of your patients is your Higher Standard. Continue to meet the emerging needs of new patient populations with SAPIEN 3 Ultra TAVI.
Click each to expand
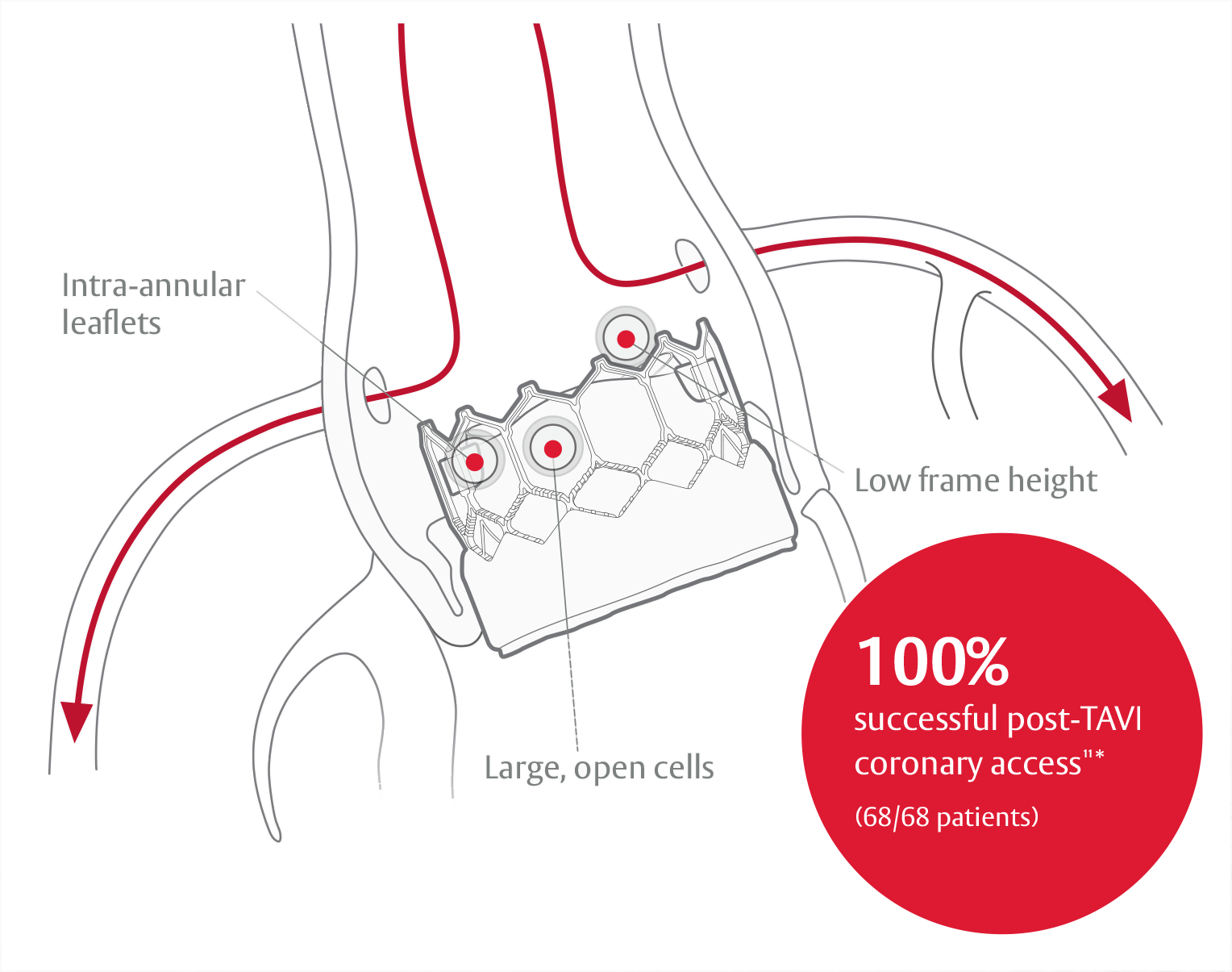
*Study only included the use of SAPIEN 3 valve.
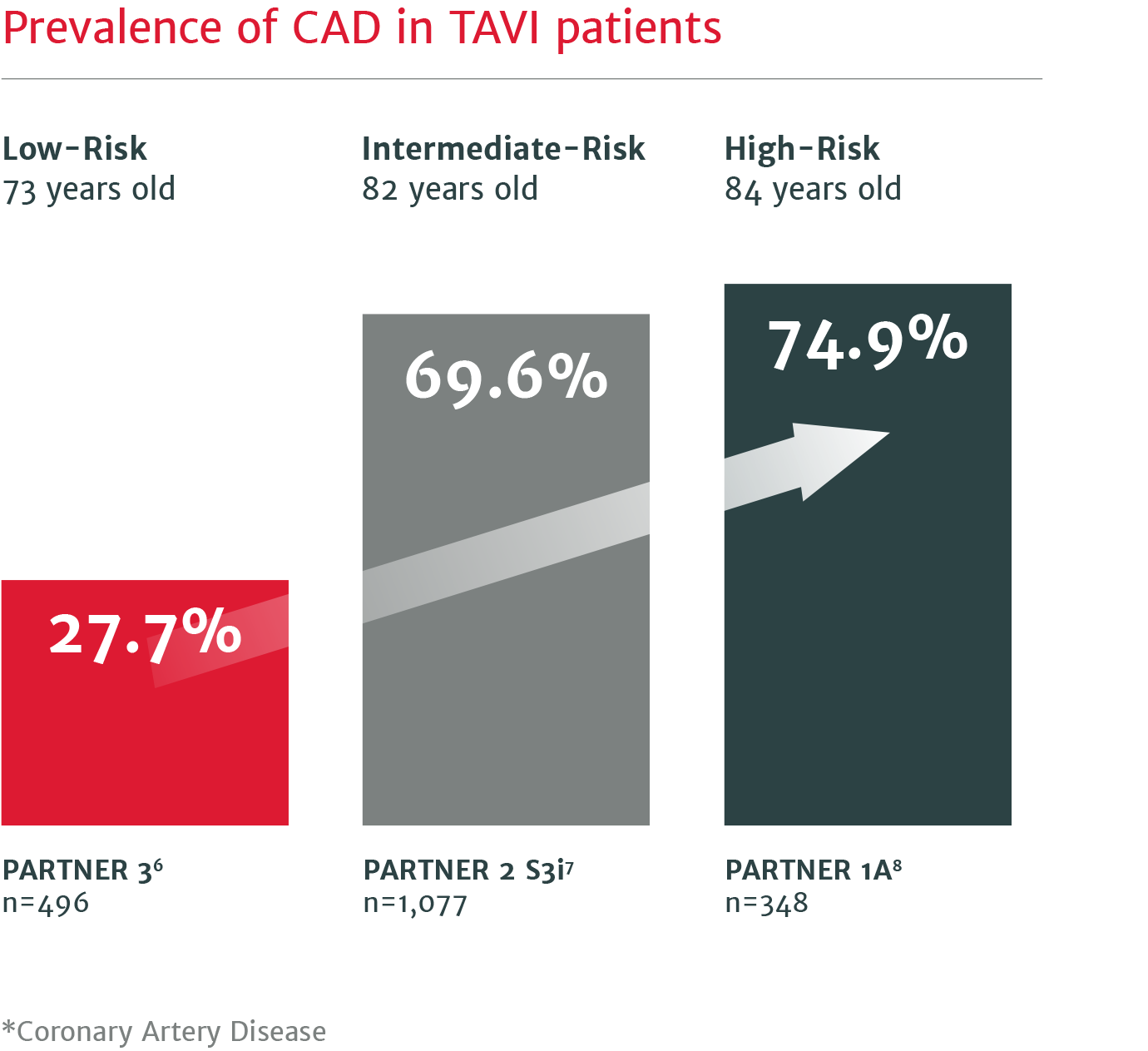
Up to 75% of severe aortic stenosis patients undergoing TAVI have CAD* requiring future coronary intervention.5
Durability that stands up to SAVR through 5 years
Low rates of SVD and SVD-related BVF at 5 years in the PARTNER II trial4
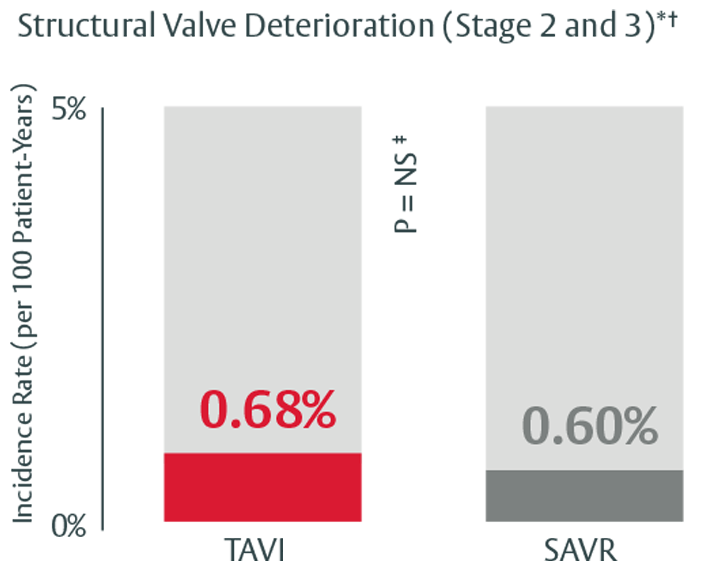
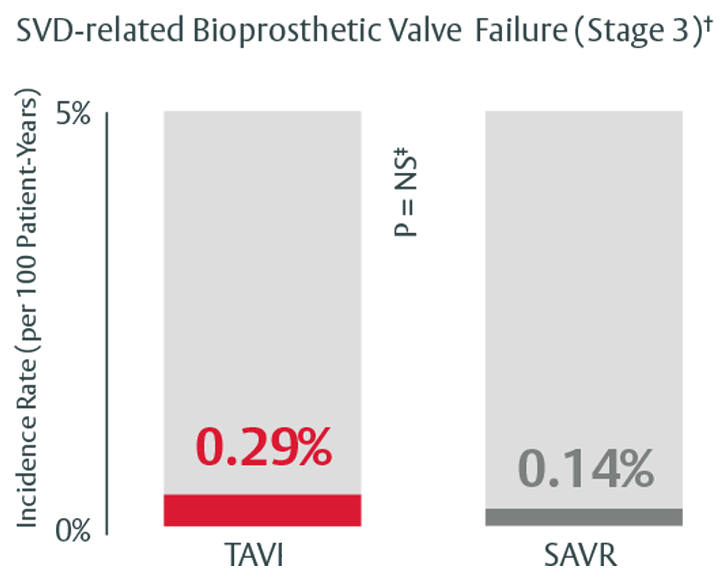
*Stage 2 & 3 (moderate & severe HVD): morphological valve deterioration AND +Δ mean gradient ≥ 10 mmHg with final mean gradient ≥ 20 mmHG§ and any of: -Δ AVA ≥ 0.3 cm2 (or ≥ 25%)§, -Δ DVI ≥ 0.1 (or ≥ 20%)§ OR ≥ 1 grade Δ transvalvular AR with final grade ≥ moderate.
†Stage 3 (severe HVD): morphological valve deterioration AND +Δ mean gradient ≥ 20 mmHG with final mean gradient ≥ 30 mmHg§ and any of: -Δ AVA ≥ 0.6 cm2 (or ≥ 50%)§, -Δ ≥ 0.2 (or ≥ 40%)§ OR ≥ 2 grade Δ transvalvular AR with severe final grade.
‡In this study, we used PARTNER II propensity matched data to analyze SVD rates, there was no statistically significant difference between SAPIEN 3 TAVI and SAVR for all endpoints except for all-cause BVF. The majority of cases within all-cause BVF in SAVR were due to endocarditis, while in TAVI they were due to paravalvular AR, a form of nonstructural valve dysfunction.
§Compared to echocardiographic assessment performed 1 to 3 months post-procedure (or discharge if not available).
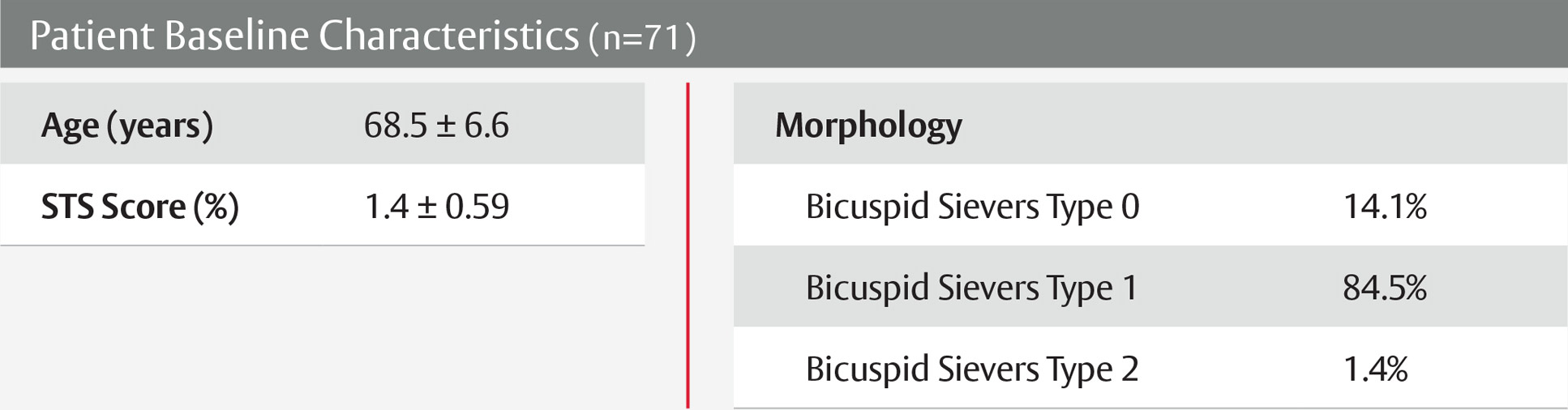
View next topicThe SAPIEN 3 valve shows excellent outcomes in low-risk bicuspid patients9
PARTNER 3 trial low-risk bicuspid nested registry
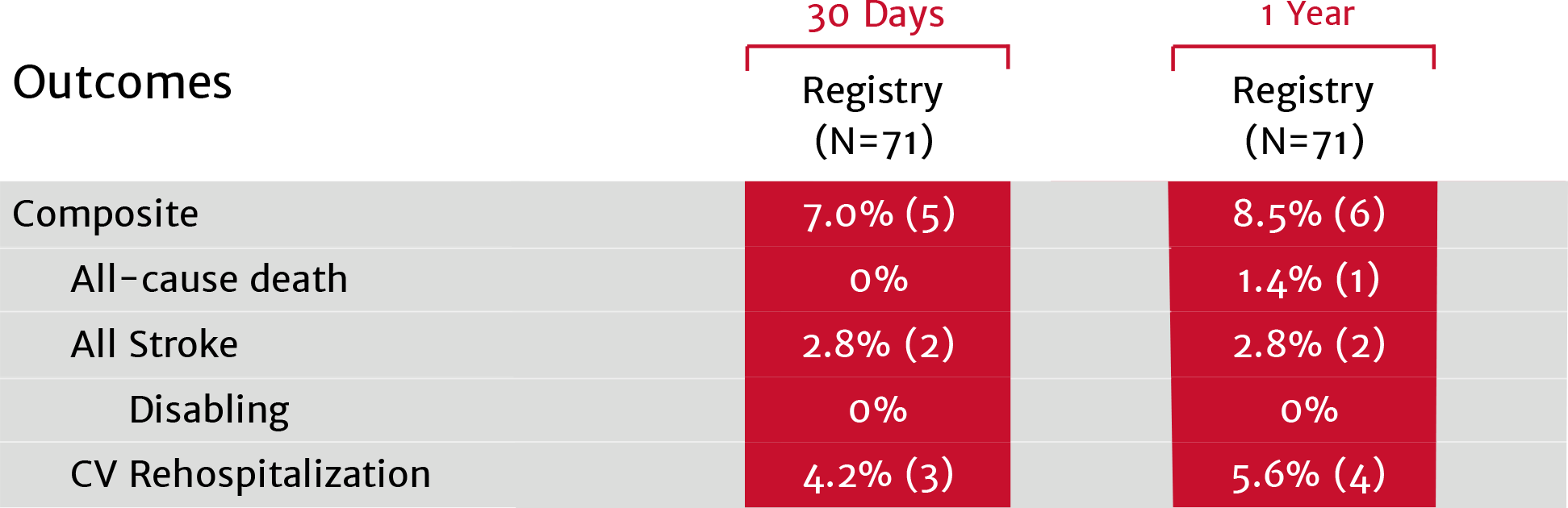
No moderate or severe PVL at 1 year9
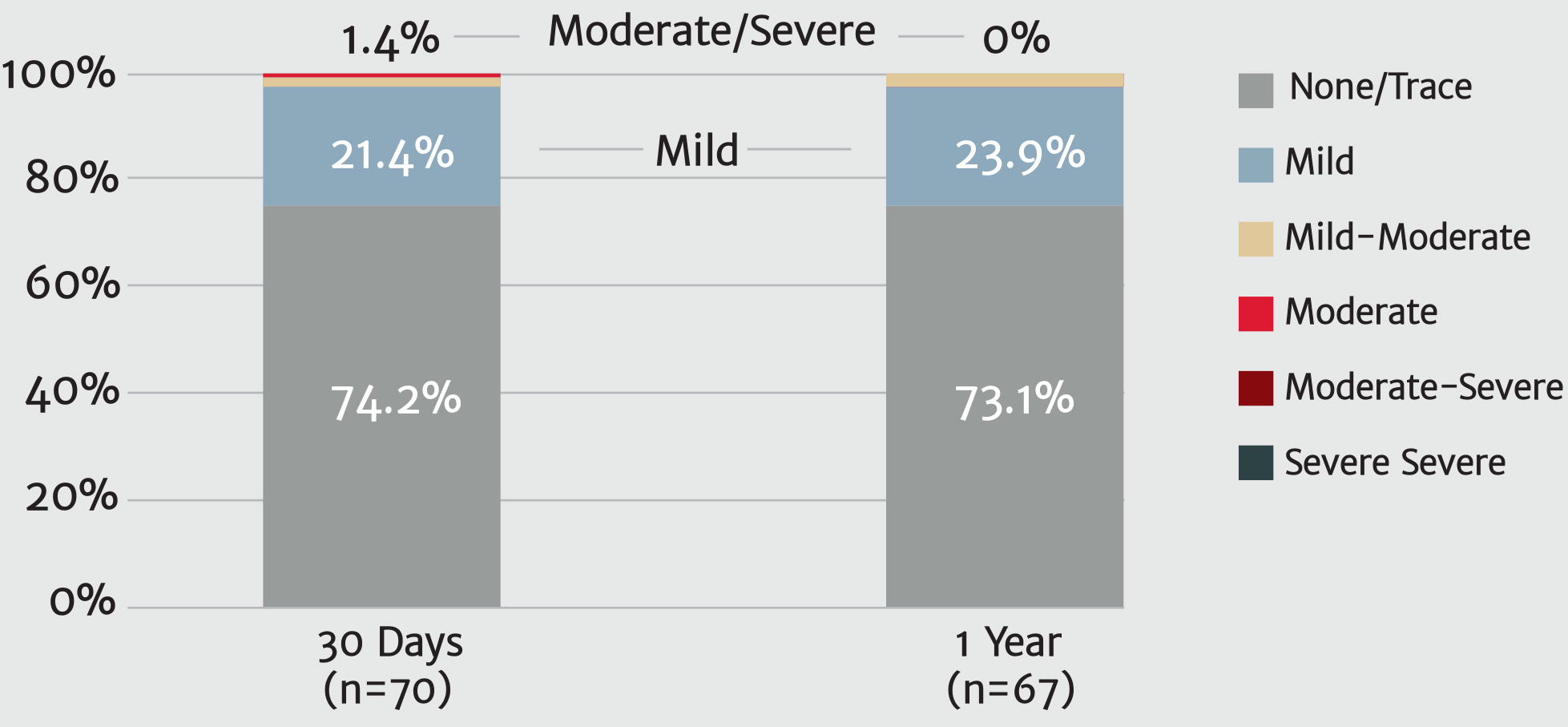
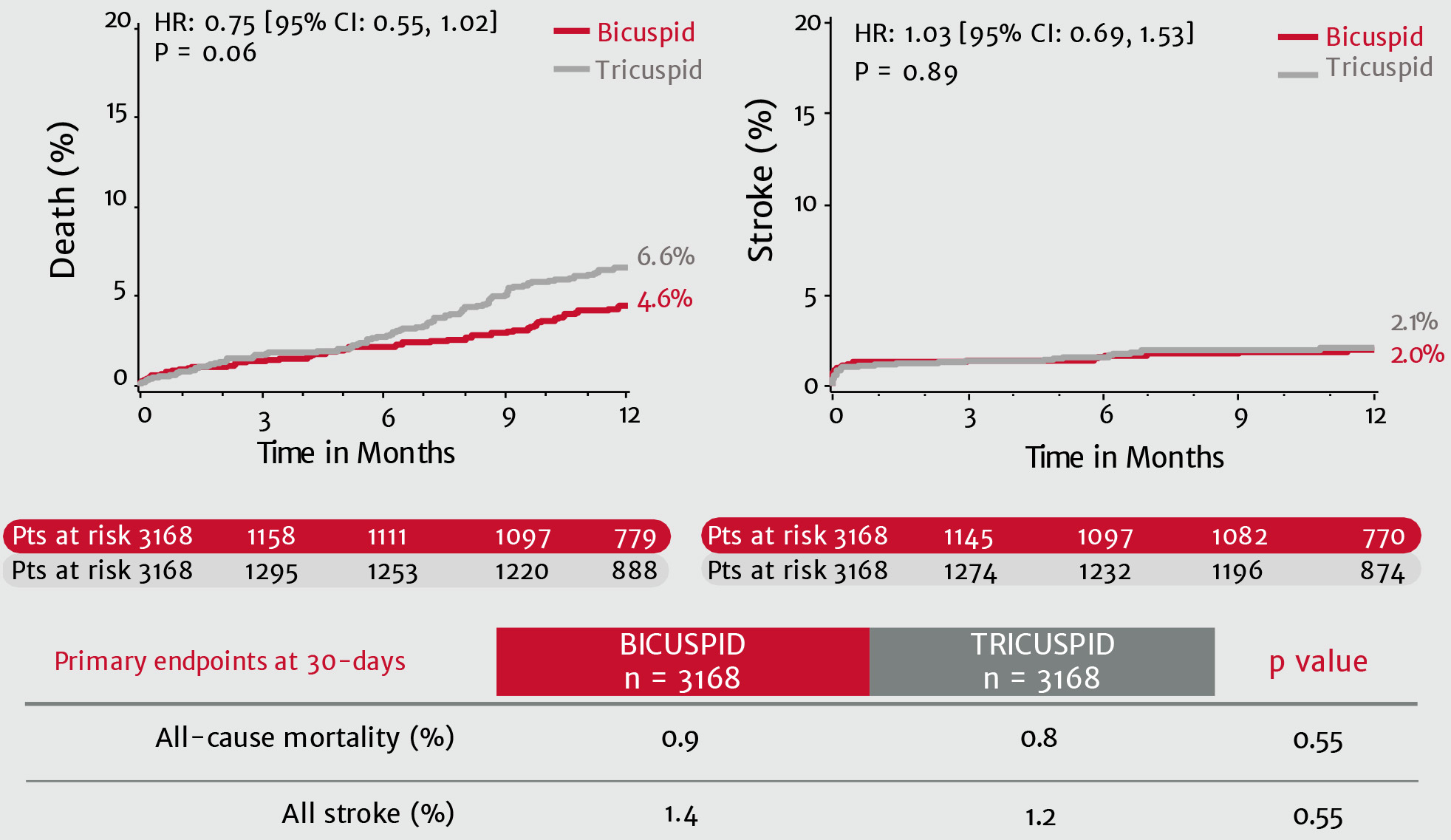
Real world SAPIEN 3 TAVI in 3,100+ bicuspid patients: No difference in mortality or stroke at 1 year compared to tricuspid patients10
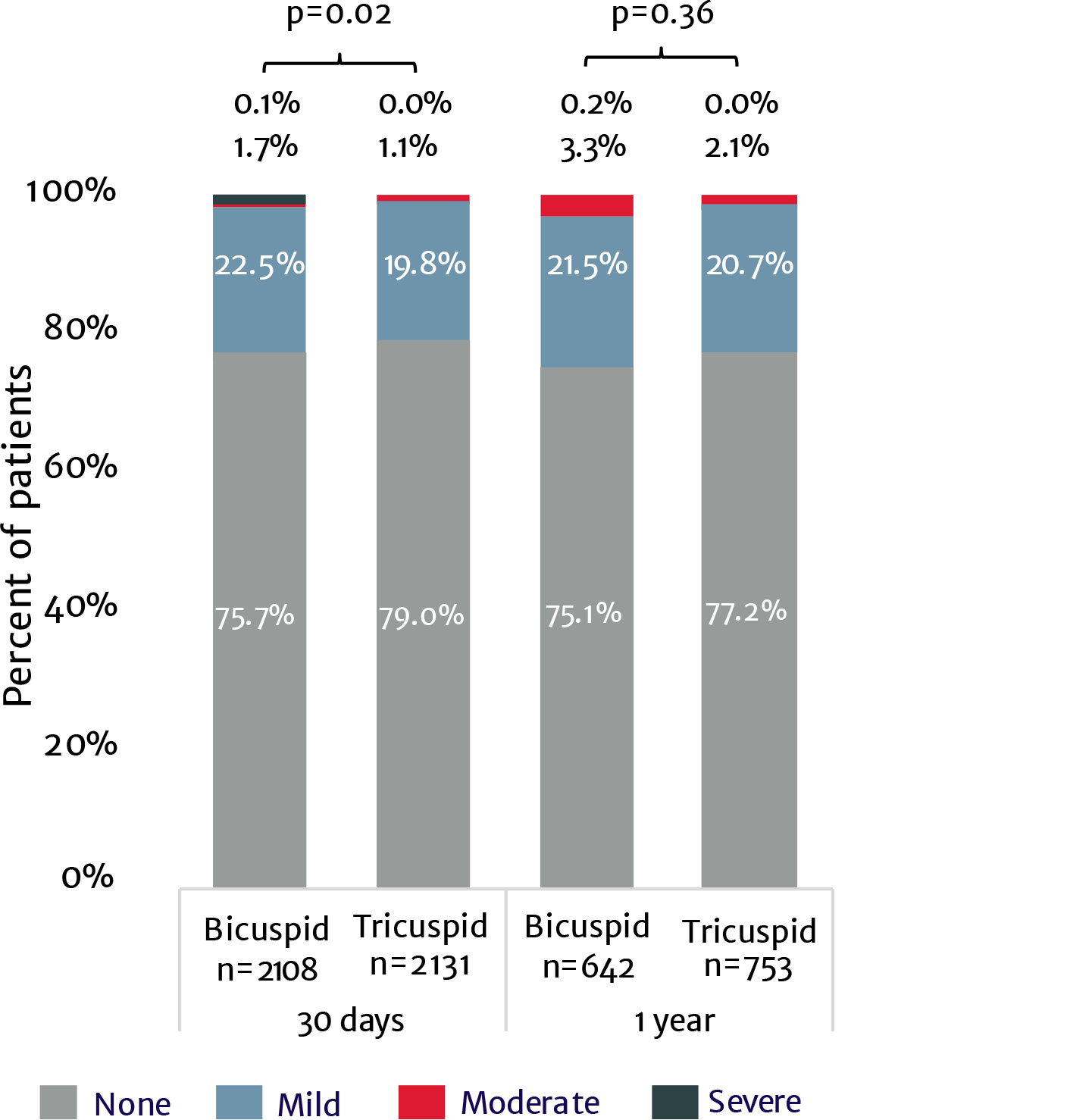
Paravalvular regurgitation
 View next topic
View next topic
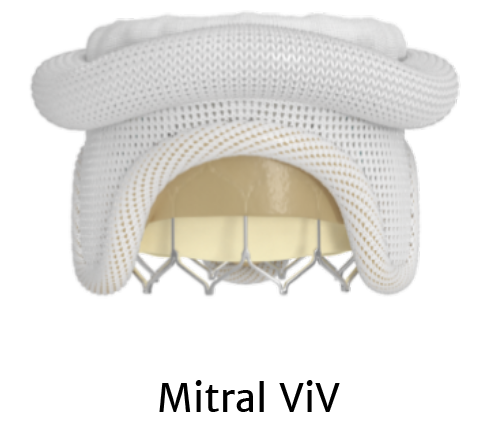
SAPIEN 3 Ultra is approved for THV-in-THV and mitral valve-in-valve.
Treatment options with SAPIEN 3 TAVI

References: 1. Ochiai T, Chakravarty T, Yoon SH, et al. Coronary access after TAVR. JACC Cardiovasc Interv. 2020;13(6):693-705. 2. De Backer O, Landes U, Fuchs A, et al. Coronary access after TAVR-in-TAVR as evaluated by multidetector computed tomography. JACC Cardiovasc Interv. 2020; 13(21):2528-2538. 3. 2017 ACS Census Data, 2018 Medicare Quarterly SAF. 4. Pibarot, et al. Structural Deterioration of Transcatheter Versus Surgical Aortic Valve Bioprostheses in PARTNER II Trial. J Am Coll Cardiol. 2020. 5. Yudi MB, Sharma SK, Tang GHL, Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71(12):1360-1378. 6. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expendable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. 7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. 8. Leon MB, Smith CR, Mack MJ, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. 9. Williams M.et al. The PARTNER 3 Bicuspid Registry for SAPIEN 3 TAVR in Low Surgical Risk Patients; Presented at TCT 2020; October 202, tctconnect.com. 10. Makkar RR, et al. Outcomes of Transcatheter Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis in the Low-Surgical Risk Population. EuroPCR 2021 May. 11. Tarantini G, Fovino LN, Le Prince P, et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon-expandable valve. Circ Cardiovasc Interv. 2020;13:e008972. DOI: 10.1161/CIRCINTERVENTIONS.120.008972.