Delivering the
Outcomes You Demand
Elevating expectations to reach your higher TAVI standards.
Delivering the
Outcomes You Demand
Elevating expectations to reach your higher TAVI standards.
Only SAPIEN 3 TAVI is proven superior to surgery in low-risk patients1
PARTNER 3 Low-Risk Trial
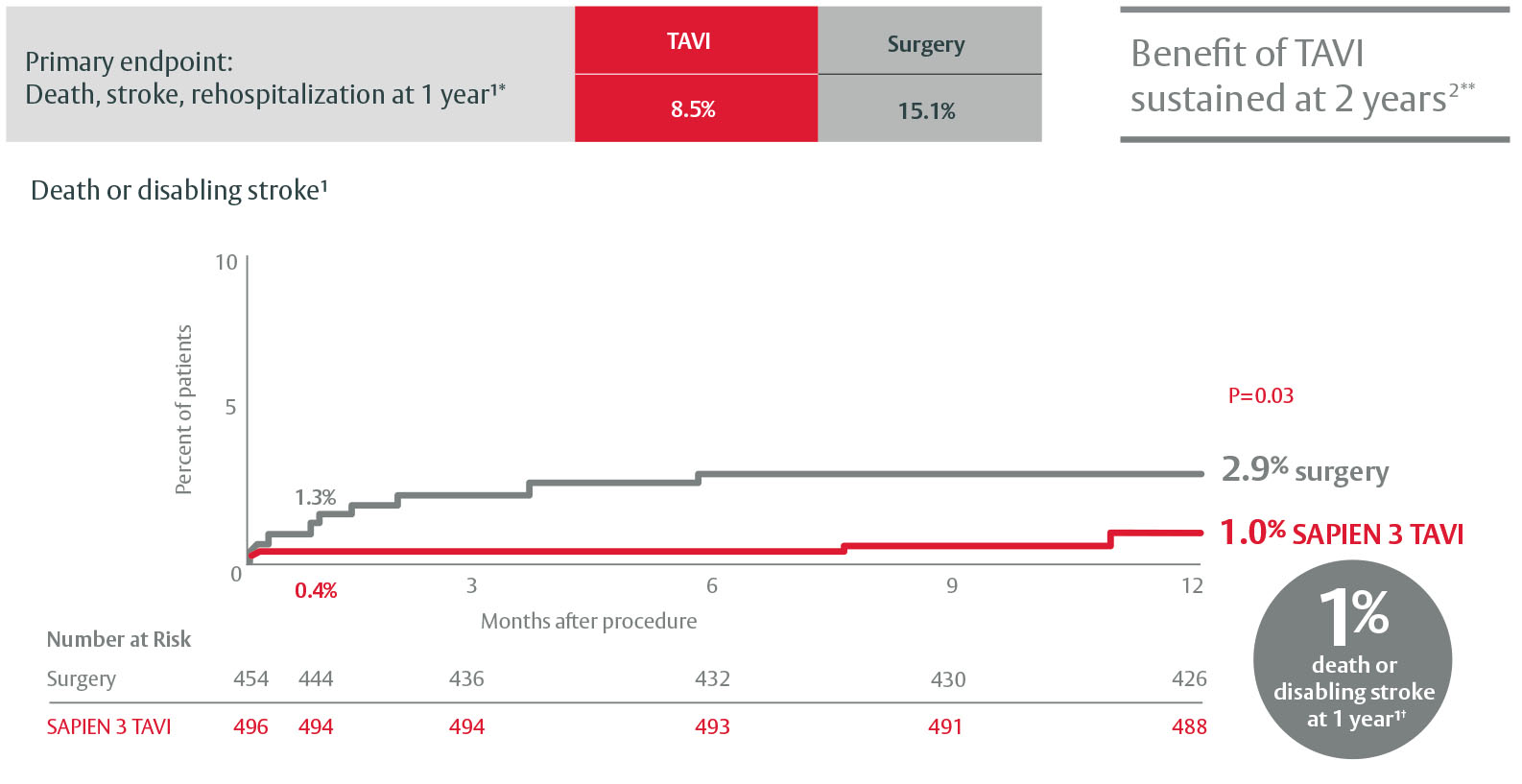
*As defined in the PARTNER 3 trial: valve related, procedure related, cardiac related rehospitalization.
**More death and stroke events in TAVI patients from 1 to 2 years; no significant differences at 2 years.
†These endpoints were not subject to multiplicity adjustment.
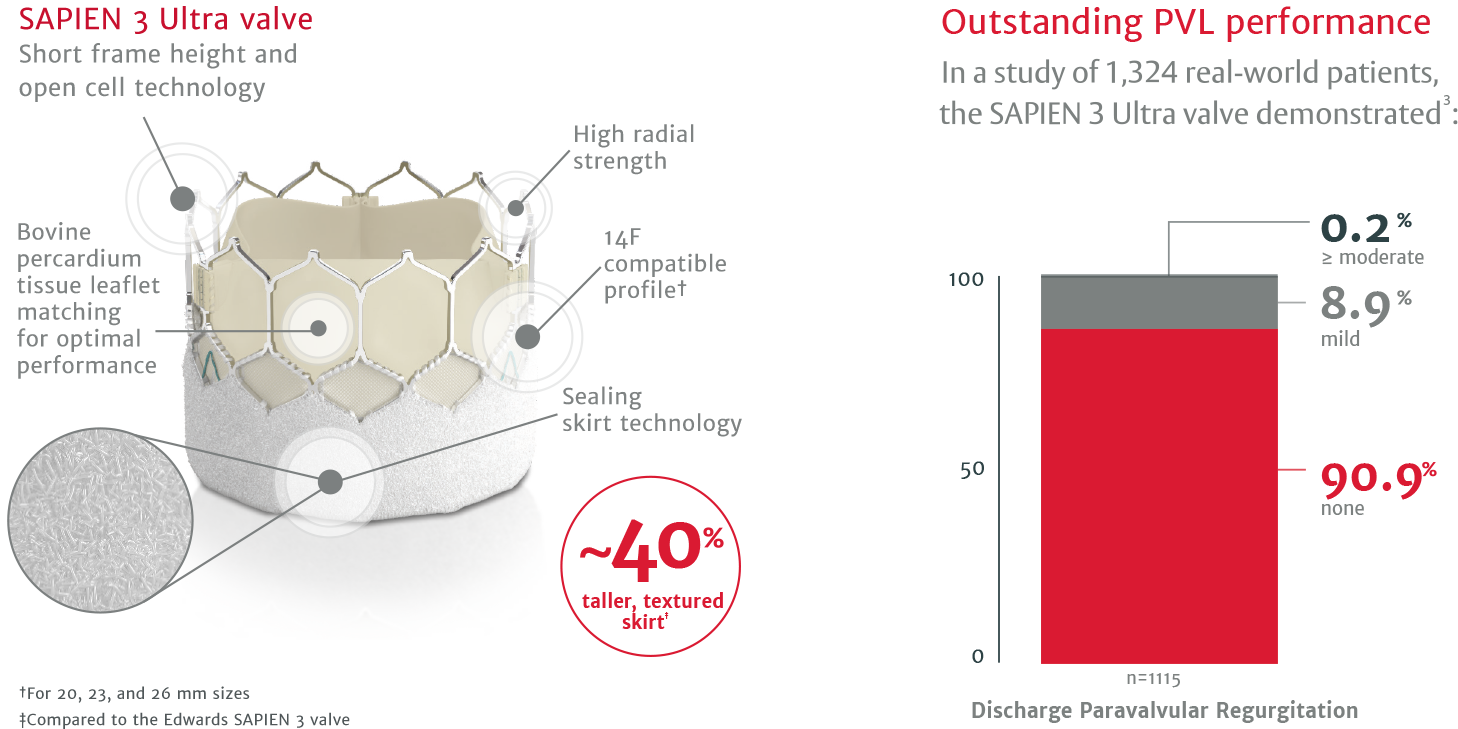
Differentiated outcomes start with differentiated design
The SAPIEN 3 Ultra valve is built on the proven SAPIEN 3 platform.
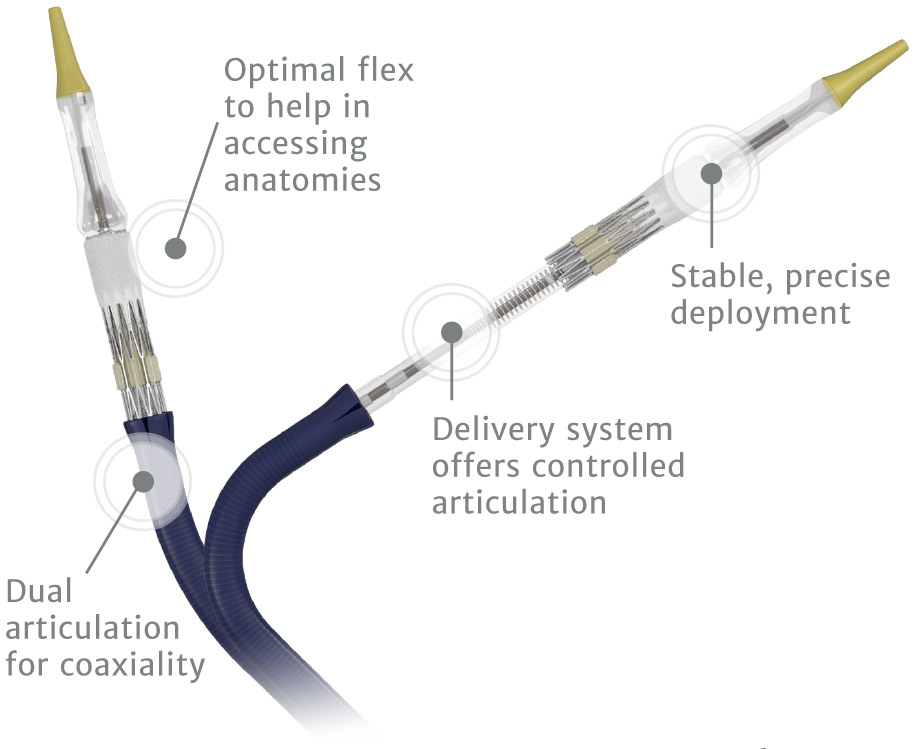
Edwards Commander delivery system
Predictability and control to meet the increasing complexity of your procedures.
stroke rate at
30 days in
low risk patients
deployment
accuracy
Single-digit new permanent pacemaker rates in low-risk patients1
at 30 days
at 1 year
References: 1. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. 2. Leon MB, Mack MJ, Hahn RT, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. JACC 2021; 779(9):1149-1161. 3. Nazif T, Daniels D, McCabe J, Chehab B, et al. Real-world experience with the SAPIEN 3 Ultra TAVR: A propensity matched analysis from the United States. Presented virtually at TVT Connect 2020. 4. PARTNER II Trial intermediate-risk cohort 30-day unadjusted clinical event rates for TAVR with the SAPIEN 3 valve, AT population (n=1077).