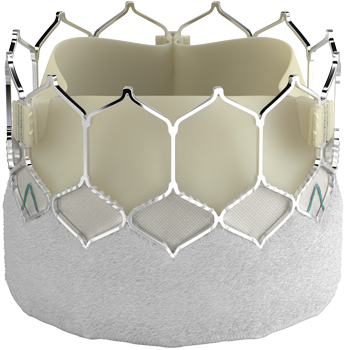
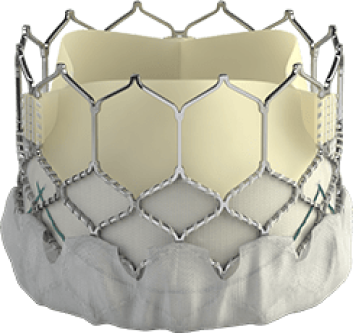
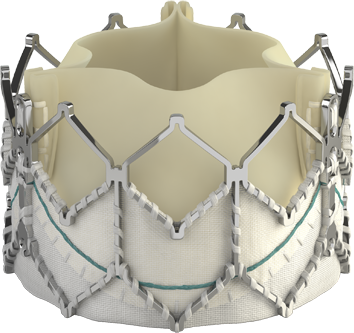
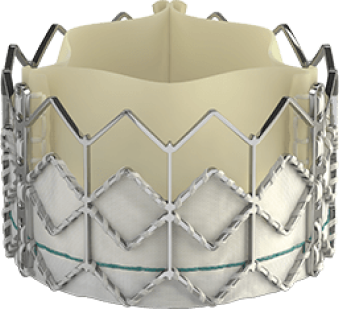
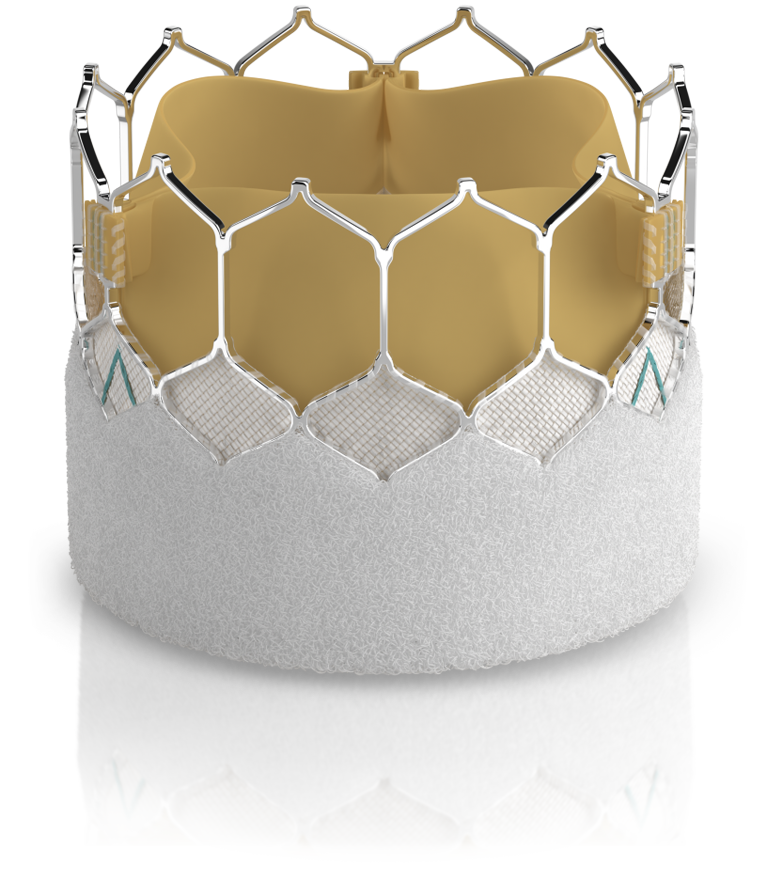
THV = Transcatheter Heart Valves
TAVI = Transcatheter Aortic Valve Implant
No clinical data are available to evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
References:
1. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation. 2002;106:3006-3008.
2. Weigerinck EMA, Van Kesteren F, Mourik MS, et al. An up-to-date overview of the most recent transcatheter implantable aortic valve prostheses. Expert Rev Med Devices 2016;13(1):31-45.
3. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607.
4. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med. 2011;364:2187-2198.
5. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
6. Mack M, Leon M, Thourani R, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
7. Nazif TM, et al. Real-World Experience With the SAPIEN 3 Ultra Transcatheter Heart Valve: A Propensity-Matched Analysis From the United States. Circ Cardiovasc Interv. 2021 Sep;14(9):e010543.
8. Saia F, et al. In-hospital and thirty-day outcomes of the SAPIEN 3 Ultra balloon-expandable TAVR: the S3U registry. Eurointervention 2020;15(14):1240-1247.
9. Data on file at Edwards Lifesciences.
10. De la Fuente AB, Wright GA, Olin JM, et al. Advanced Integrity Preservation Technology Reduces Bioprosthesis Calcification While Preserving Performance and Safety. Jrnl of Heart Valve Disease. 2015.
11. Tarantini G et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon-expandable valve. Circ Cardiovasc Interv. 2020.