Your first move defines the next
A unique design built for consistent outcomes
Your first move defines the next
A unique design built for consistent outcomes
Consistently demonstrating the results you need for the outcomes that matter1,9
The Edwards SAPIEN 3 valve design has revolutionized how we treat severe aortic stenosis today, leveraging decades of Edwards surgical valve innovation
Building on a legacy of superior outcomes
Proven in the landmark PARTNER clinical trials
- Only SAPIEN 3 valve is proven superior to surgery* in low-risk patients
- 8.5% death, stroke, rehospitalization versus 15.1% for surgery at 1 year*1
*In the PARTNER 3 trial, SAPIEN 3 TAVI was proven superior to surgery on the primary endpoint of all-cause death, all stroke, and rehospitalization (valve-related or procedure-related, and including due to heart failure) at one year and multiple pre-specified secondary endpoints in low-risk patients.
Supported by outstanding outcomes, the SAPIEN 3 platform delivers at 1 year:1
Consistently strong outcomes for a variety of patient morphologies:
- Excellent clinical outcomes in low-risk bicuspid patients2
- Consistently strong outcomes across indicated annular sizes3
The Edwards SAPIEN platform brochure
When it comes to lifetime management, learn more about how the Edwards SAPIEN 3 platform is designed to deliver a TAVI experience an implanter can count on, starting with the index procedure.
Designed to deliver a TAVI experiencea Heart Team can count on
Edwards Commander delivery system
Predictability and control to meet the increasing complexity of your procedures.4
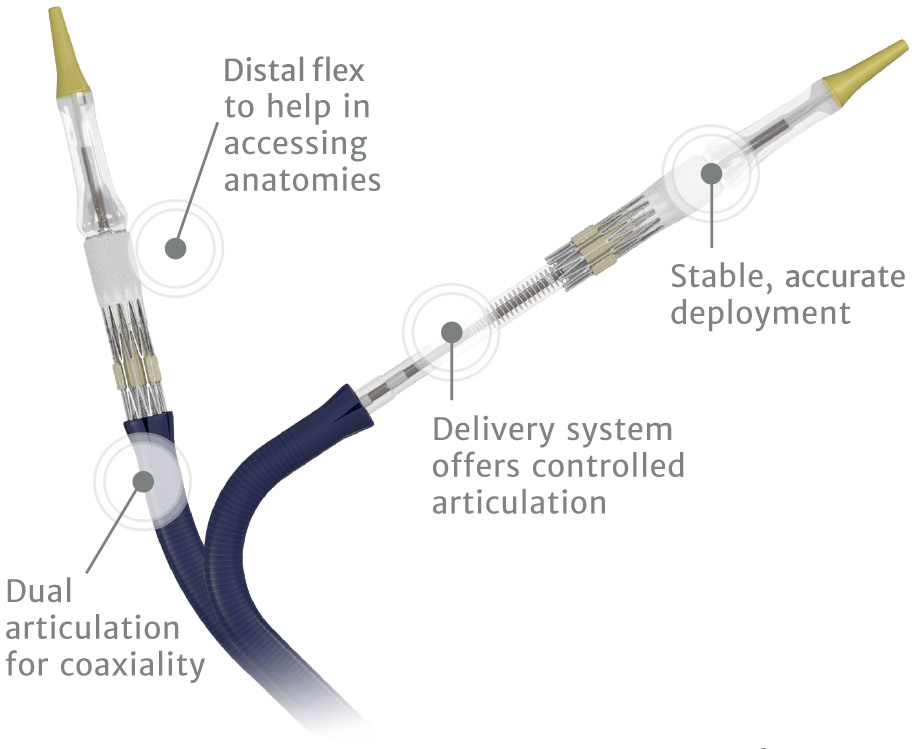
Instructions for use available on www.eifu.edwards.com.
Edwards eSheath+ introducer set5
Low profile and expandable sheath design
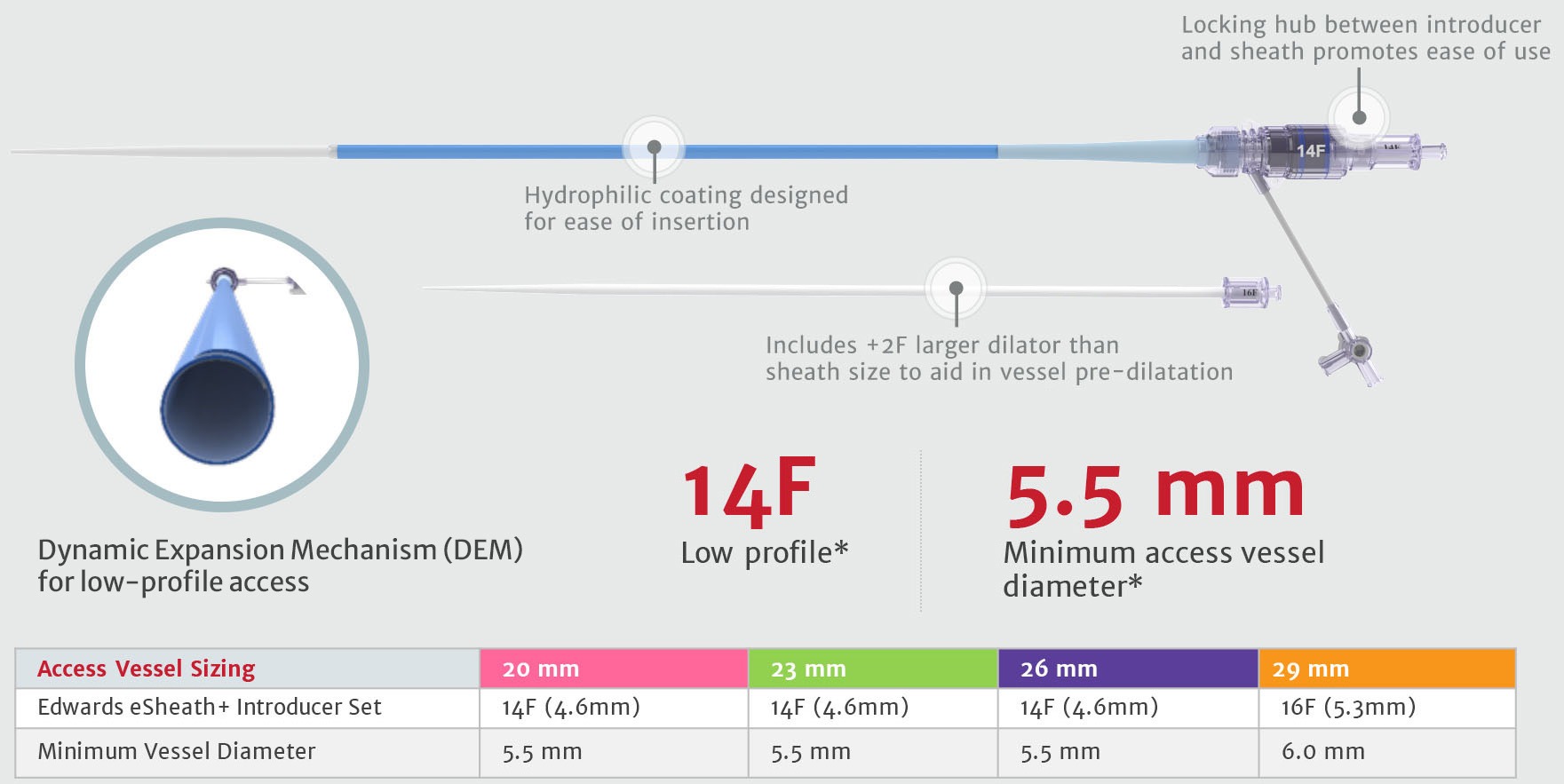
*16F & 6.0mm for 29mm SAPIEN 3 Valve



No clinical data are available to evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
References:
1. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
2. Williams MR, Jilaihawi H, Makkar R, et al. The PARTNER 3 bicuspid registry for transcatheter aortic valve replacement in low-surgical-risk patients. J Am Coll Cardiol Intv. 2022;15(5):523-32.
3. Eng MH, Abbas AE, Hahn RT, et al. Real world outcomes using 20 mm balloon expandable SAPIEN 3/ultra valves compared to larger valves (23, 26, and 29 mm)- a propensity matched analysis. Catheter Cardiovasc Interv. 2021;98(6):1185-92.
4. Thourani VH, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016 May 28;387(10034):2218-25.
5. Edwards Lifesciences SAPIEN 3/Ultra valve Instruction for Use.
6. Pibarot P, Ternacle J, Jaber WA, et al. Structural deterioration of transcatheter versus surgical aortic valve bioprosthesis in PARTNER-2 trial. J Am Coll Cardiol. 2020;76:1830-1843.
7. Tarantini G, Nai Fovino L, Le Prince P, et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon expandable valve. Circ Cardiovasc Interv. 2020;13(7):e008972.
8. Tarantini G, et al. Redo-Transcatheter aortic valve implantation using the SAPIEN 3/Ultra transcatheter heart valves – Expert Consensus on Procedural Planning Techniques, The American Journal of Cardiology, 2023.
9. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Commander, Edwards Commander Edwards SAPIEN, Edwards SAPIEN 3, Edwards SAPIEN 3 Ultra, Edwards eSheath, eSheath, SAPIEN, SAPIEN 3, RESILIA, SAPIEN 3 Ultra, PARTNER, PARTNER II, and PARTNER 3 are trademarks or service marks of Edwards Lifesciences Corporation affiliates. All other trademarks are the property of their respective owners.
PP--EU-7518 v2.0