Introducing the
SAPIEN 3 Ultra
RESILIA Valve
Introducing the
SAPIEN 3 Ultra RESILIA Valve
Introducing the
SAPIEN 3 Ultra
RESILIA Valve
Introducing the
SAPIEN 3 Ultra RESILIA Valve
The Edwards SAPIEN 3 Ultra RESILIA valve
Effectively addressing calcification, a leading cause of tissue valve failure.*2
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Taller, textured skirt technology to reduce PVL*5
Scroll / swipe side to side to review valve line-up
.png)
*Compared to SAPIEN 3 valve
PVL = Paravalvular Leak
RESILIA tissue reduced calcification when compared to traditional surgical valve tissue treatment*
Delivering the potential to improve valve longevity and reduce reintervention
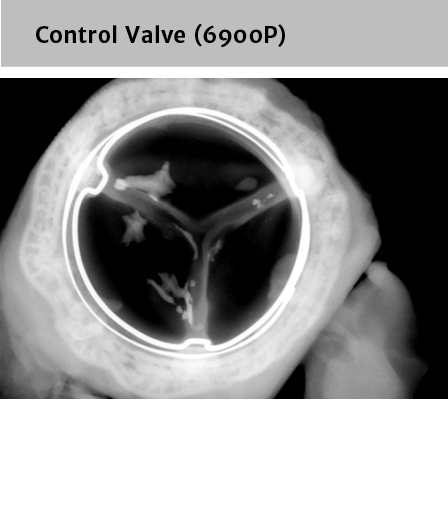
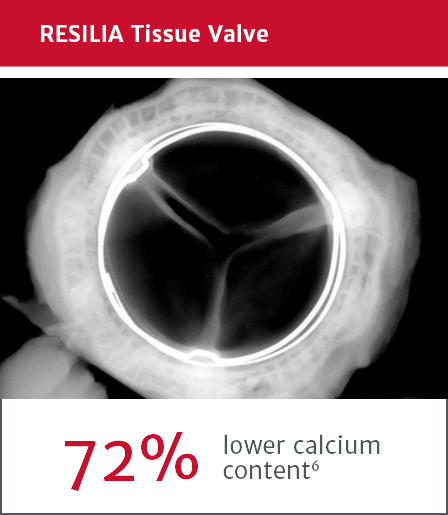
RESILIA tissue showed significant improvement in calcium-blocking properties*
*RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards Lifesciences in a juvenile sheep model.
RESILIA tissue showed excellent and consistent outcomes over 7 years7
RESILIA tissue data from the COMMENCE aortic trial* show:
*Includes the following valve sizes: 19 mm, 21 mm, 23 mm, 25 mm, 27 mm, 29 mm.
†*SVD: Structural Valve Deterioration defined as dysfunction or deterioration involving the operated valve (exclusive of infection or thrombosis), as determined by reoperation, autopsy or clinical investigation with periodic echocardiography; SVD refers to valve intrinsic changes, such as wear and tear, fracture, calcification, etc. (Akins et al, 2008). SVD was adjudicated by independent committee.
The ultimate lifetime management solution for all eligible patients



Download information and clinical data
No clinical data are available to evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
References:
1. Data on file.
2. De la Fuente et al. Advanced Integrity Preservation Technology Reduces Bioprosthesis Calcification While Preserving Performance and Safety. Journal of Heart Valve Disease. 2015.
3. Kodali S et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards SAPIEN valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015.
4. Makkar R et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020.
5. Stinis CT, Abbas AE, Teirstein P, et al. Real-World Outcomes for the Fifth-Generation Balloon Expandable Transcatheter Heart Valve in the United States. JACC Cardiovasc Interv. 2024.
6. Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015.
7. Beaver T, Bavaria J, Griffith B, et al. Seven-Year Outcomes Following Aortic Valve Replacement with a Novel Tissue Bioprosthesis. Journal of Thoracic and Cardiovascular Surgery. 2023.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, Edwards SAPIEN, Edwards SAPIEN 3, Edwards SAPIEN 3 Ultra, PARTNER, RESILIA, SAPIEN, SAPIEN 3, and SAPIEN 3 Ultra are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
PP--EU-8423 v1.0