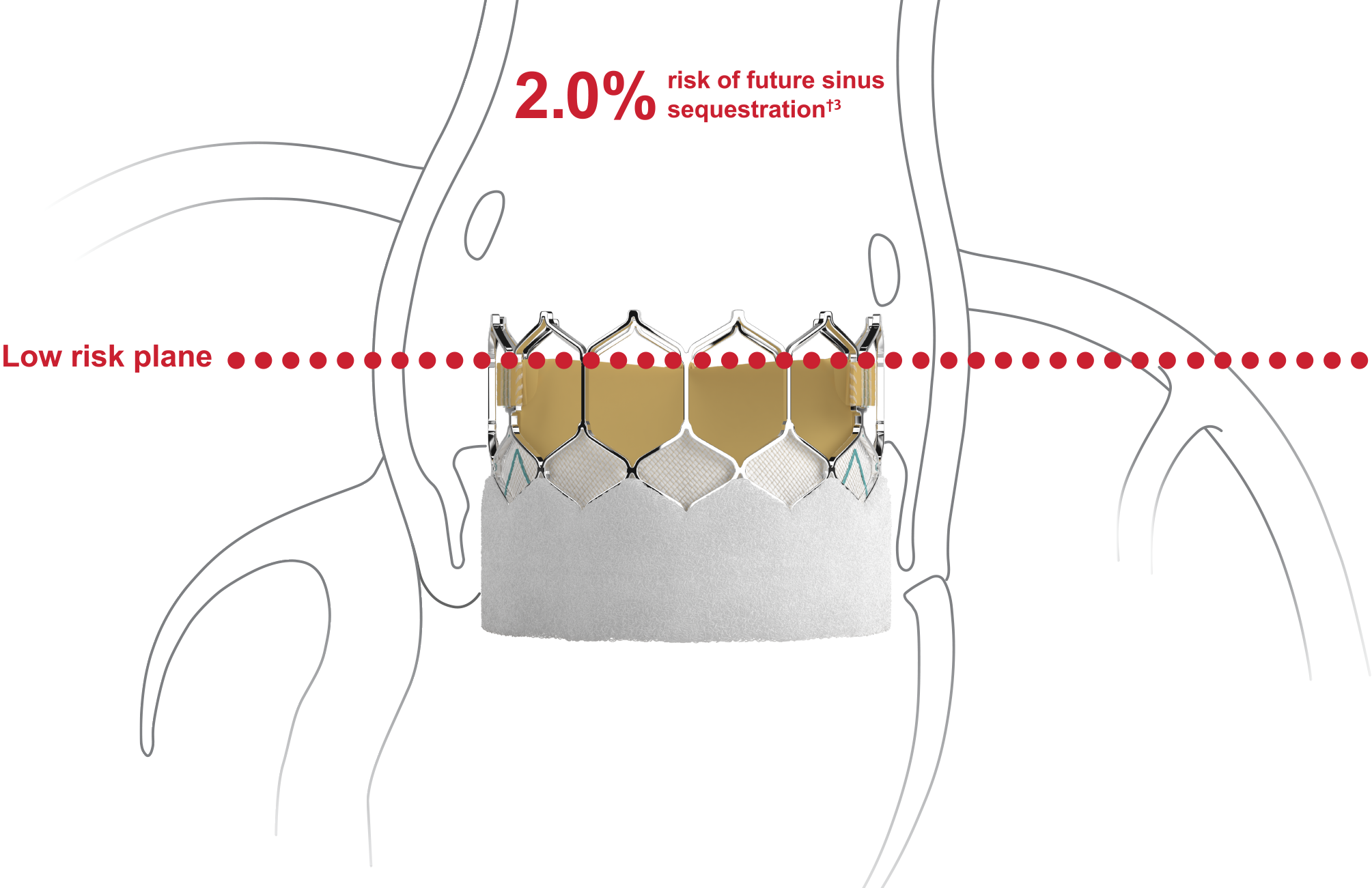
Designed to host future valve interventions
Preserving future coronary access is a key consideration in valve-in-valve patients3,4
- SAPIEN 3 valve-first TAVI reduces risks of future sinus sequestration†2
- SAPIEN 3 valve-first TAVI facilitates favorable coronary access3
†Risk of sinus sequestration if (1) prior TAV commissure level above STJ and (2) the distance between TAV and STJ was <2.0mm in each coronary sinus.