Uses same trusted bovine pericardium leaflet and ThermaFix anticalcification process as the Edwards SAPIEN 3 valve.
Edwards SAPIEN 3 Ultra valve
Built on proven technology
Edwards SAPIEN 3 Ultra valve
Built on proven technology
Advancing valve design
Built upon the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve is designed with your patient's future needs in mind.

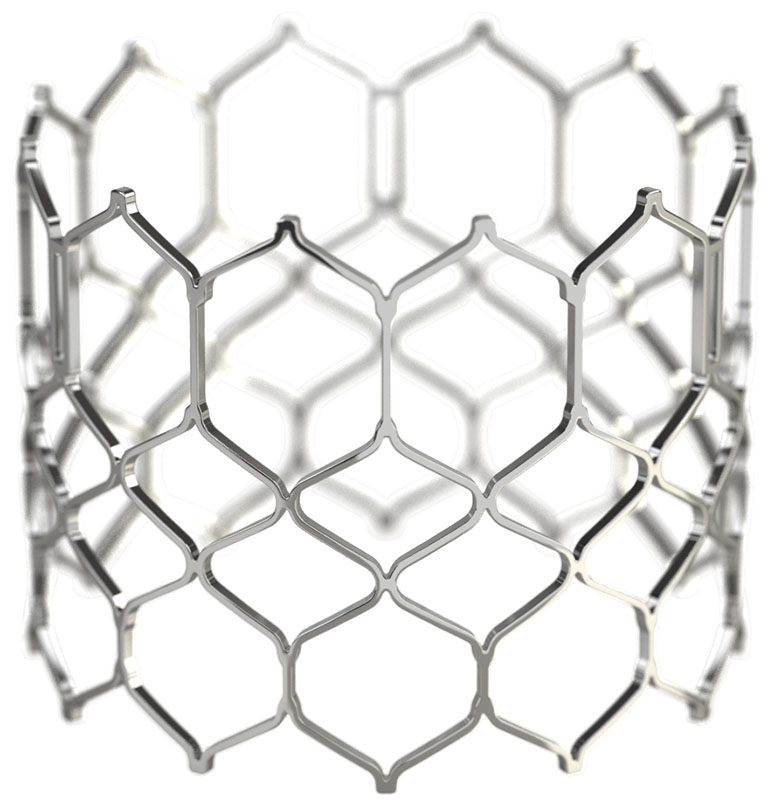
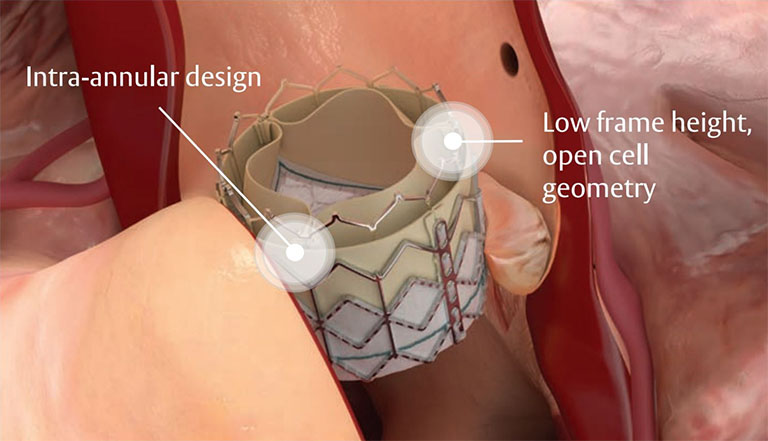
geometric frame
Low profile and open cell geometry facilitate coronary access.1
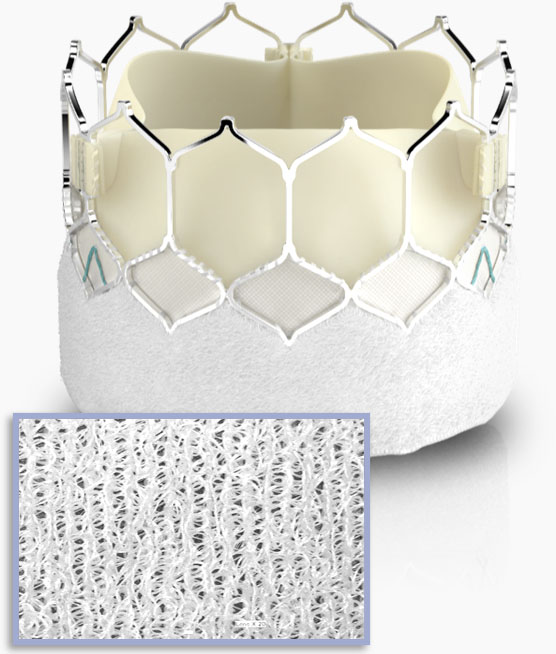
Textured fabric
technology
Features a ~40% taller, textured polyethylene terephthalate (PET) outer skirt.*
*Compared to the Edwards SAPIEN 3 valve
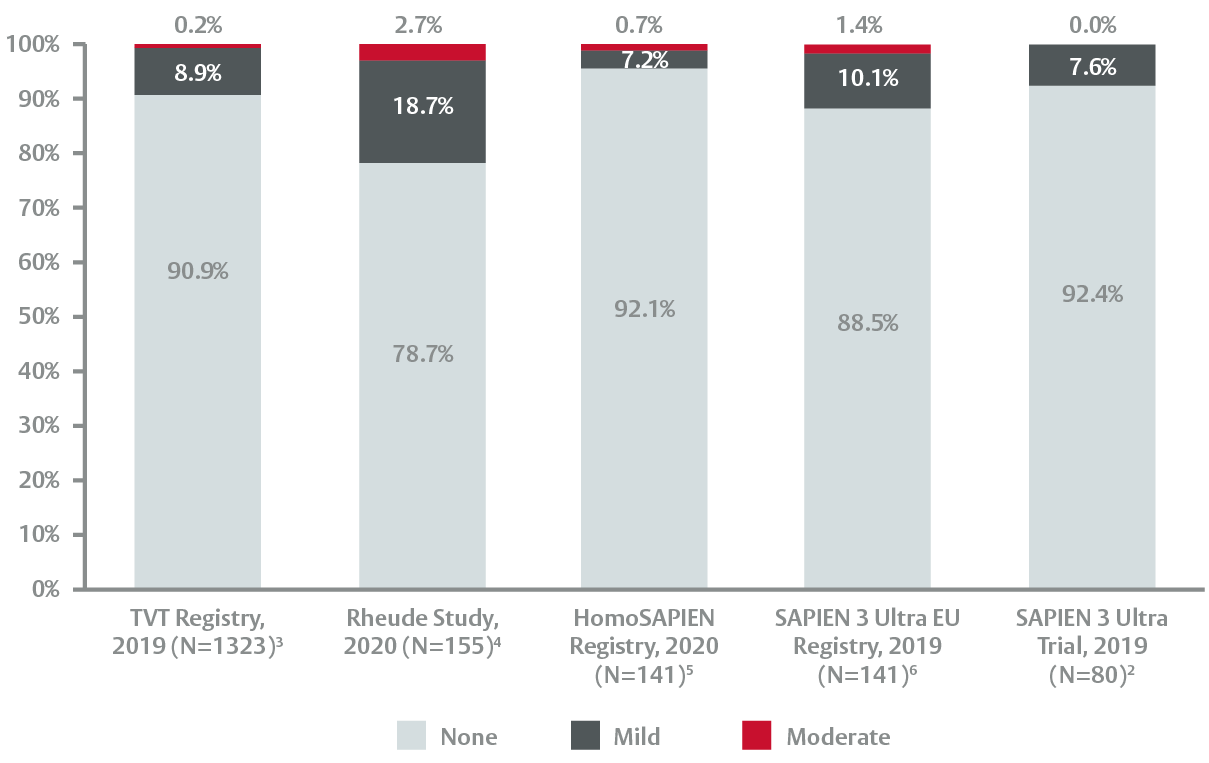
Minimising paravalvular leak2
Important reductions in paravalvular leak (PVL)
Edwards SAPIEN 3 Ultra valve engineered to further reduce PVL
In the TVT Registry in over 1300 procedures:
- Edwards SAPIEN 3 Ultra valve eliminated severe PVL and produced close to zero levels of moderate PVL3
- Consistently low levels of mild PVL across the studies2-6
Moderate and severe PVL are independent predictors of 5-year all-cause mortality in multivariable analysis.7
Consistently low paravalvular leak rates at discharge3*
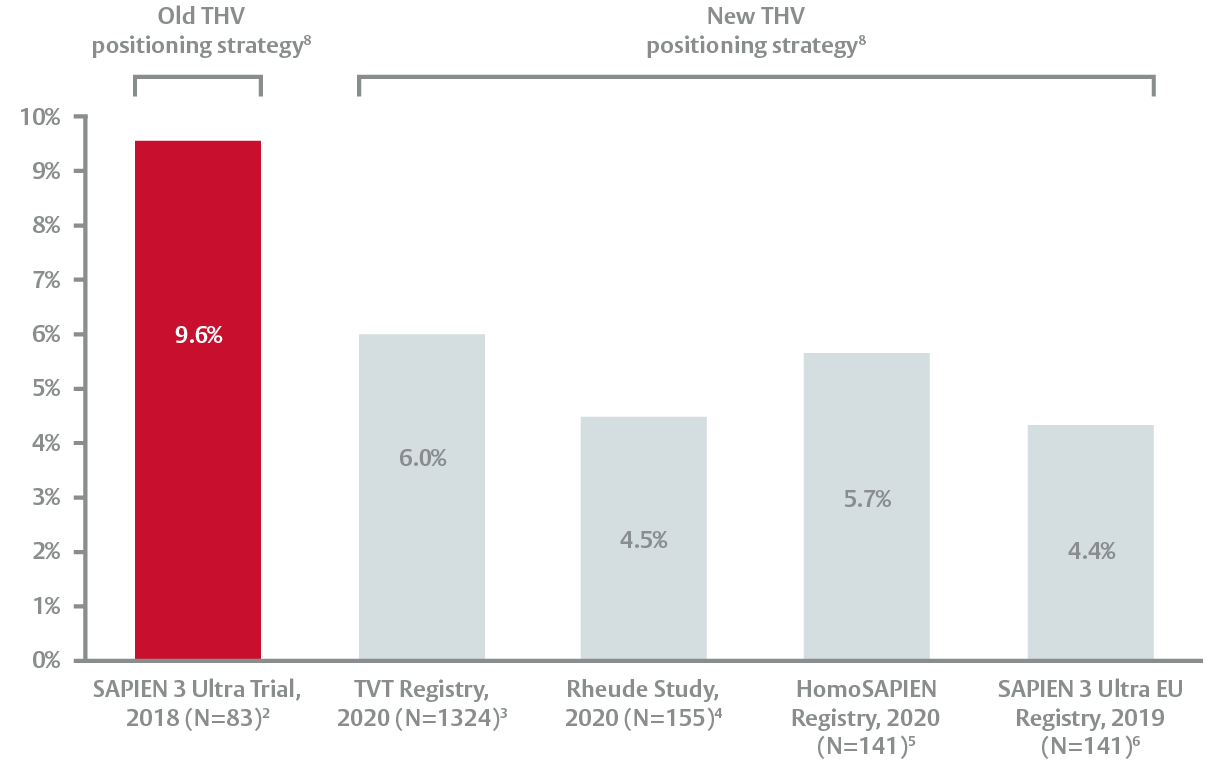
Single digit new permanent pacemaker rates
Low rates of new permanent pacemaker implantation (PPI) achieved with a high valve positioning strategy8
- New PPI is an independent predictor of mortality at 1 year9
Single digit new PPI rates at 30 days (including baseline)*
Today's transcatheter heart valve (THV) solution designed for lifetime patient management
Edwards SAPIEN 3 transcatheter aortic valve implantation (TAVI) is indicated for use in aortic and mitral redo procedures (including THV-in-THV)*
A lifesaving reintervention when TAVI is a preferred treatment option.
*For patients at high or greater surgical risk
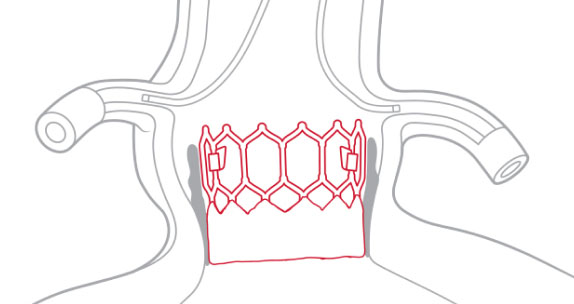
Designed for future coronary access
Low profile frame design and open geometry facilitates future coronary access.1
Registry data from SOURCE 3 showed 100% successful coronary access (n=61) post-TAVI.10

Edwards SAPIEN 3 Ultra valve Instructions for Use
See the Operator's Manual for all Edwards Lifesciences products.
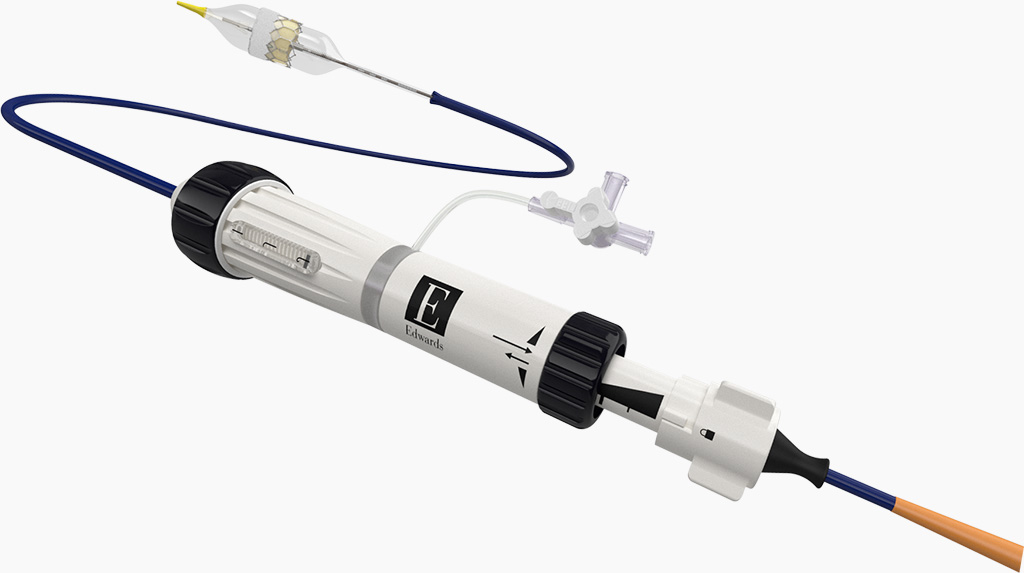
Visit Instructions for Use websiteThe Edwards COMMANDER delivery system
Designed to streamline the TAVI procedure and reduce complications with an ultra-low delivery profile, optimal positioning control and predictable deployment.
For predictable, proven results
Features of the Edwards COMMANDER delivery system:
- Stable, precise deployment delivering 99% first-time accuracy11†
- Low delivery profile
- Dual articulation for coaxiality
- Optimal positioning control
†The PARTNER II Trial intermediate-risk cohort 30-day unadjusted clinical event rates for TAVI with the Edwards SAPIEN 3 valve, as-treated population (n=1078).11
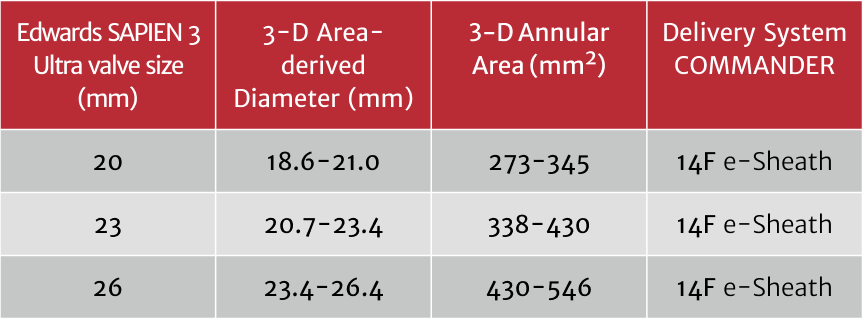
3D Sizing and Delivery System Guide
Edwards SAPIEN 3 Ultra valve is available in 3 sizes for maximum flexibility and optimal fitting.
Edwards SAPIEN 3 valve is also available in a 29mm size for larger annular diameters.
Stay informed with our newsletter
Thank you!
Thank you for signing up to the heartvalves.com newsletter. You will now receive emails on the latest developments and industry insights on heart valve innovation and technology.
References:
1. Yudi MB, et al. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71(12):1360-1378.
2. Webb, J. A prospective multicenter study of the SAPIEN 3 Ultra system in intermediate-risk patients with severe aortic stenosis. Presented at PCR London Valves 2019 (Nov 2019).
3. Nazif et al. Real-World Experience with the SAPIEN 3 Ultra TAVR: A Propensity Matched Analysis from the United States, TVT Connect 2020.
4. Rheude. Transcatheter Aortic Valve Replacement With Balloon-Expandable Valves Comparison of SAPIEN 3 Ultra Versus SAPIEN 3. JACC: Cardiovasc Interv. 2020;13(22)2631-2638.
5. Moriyama N. et al. Hemodynamic comparison of TAVR with the SAPIEN 3 Ultra versus SAPIEN 3: The HomoSAPIEN registry. Catheter Cardiovasc Interv. 2021;97(7):E982-E991.
6. Saia F et al. SAPIEN 3 Ultra balloon-expandable transcatheter aortic valve: in-hospital and 30-day results from the multicentre S3U registry. EuroIntervention. 2020;15(14):1240-1247.
7. Giuliano Costa MD et al., Long-term outcomes of self-expanding versus balloon-expandable transcatheter aortic valves: Insights from the OBSERVANT study. Valvular and Structural Heart Diseases. First published: 13 April 2021. https://doi.org/10.1002/ccd.29701
8. Sammour Y. et al. Systematic Approach to High Implantation of SAPIEN-3 Valve Achieves a Lower Rate of Conduction Abnormalities Including Pacemaker Implantation. Circulation: Cardiovascular Interventions 2021, 14:e009407.
9. Glaser N, et al., JAMA Network Open. 2021;4(7):e2116564. doi:10.1001/jamanetworkopen.2021.16564.
10. Tarantini G, Fovino LN, Le Prince P, et al. Coronary access and percutaneous coronary intervention up to 3 years after transcatheter aortic valve implantation with a balloon-expandable valve. Circ Cardiovasc Interv. 2020;13(7):e008972.
11. Thourani VH et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387: 2218–2225.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-3361 v1.0