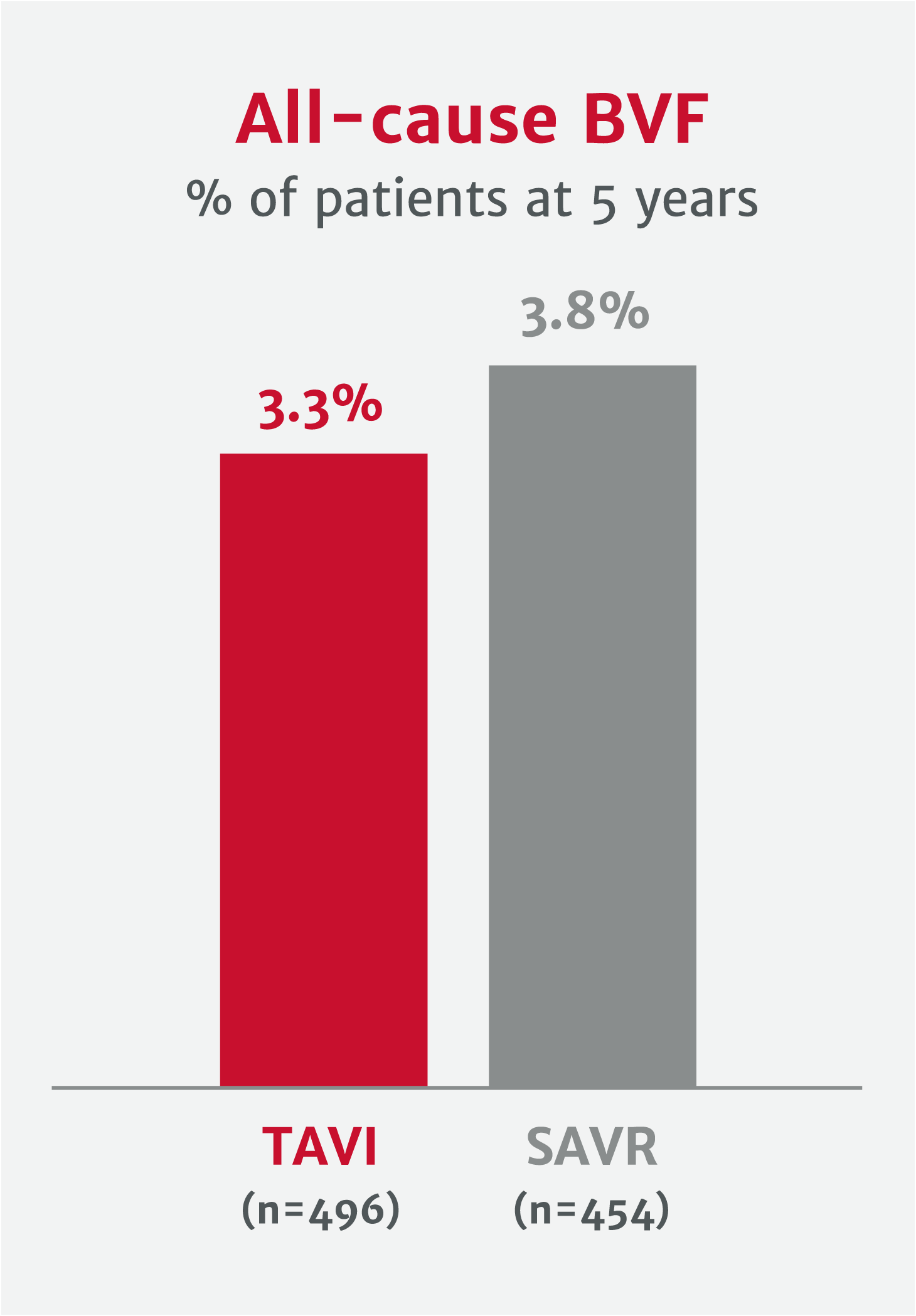The SAPIEN 3 Ultra RESILIA valve
Advanced calcium-blocking tissue technology1,2
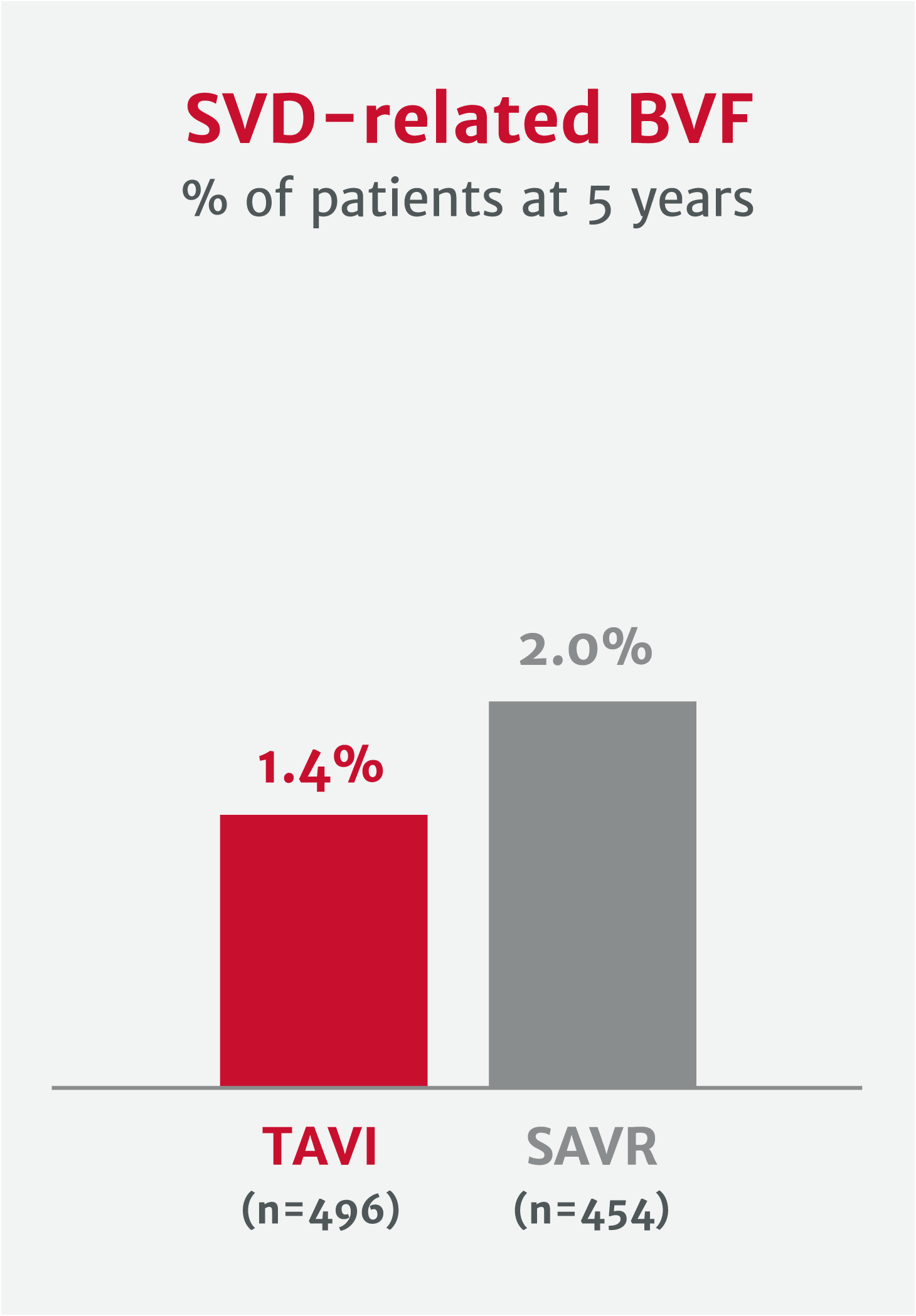
Potential to improve valve longevity and reduce risk of reintervention
Taller*, textured outer skirt extended to 29mm valve1
Delivering the PVL results you demand impacting immediate and long-term outcomes3,4
Only THV with dry tissue storage1
Mitigates calcium-attracting glutaraldehyde residuals
Explore clinical data for RESILIA tissue*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
†RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg 2015;149:340-5.