728,000 lives and counting.
We're just getting started.
728,000 lives and counting.
We're just getting started.
Innovating together, with 25,000+† patients studied around the world.
Scroll / swipe side to side to review timeline
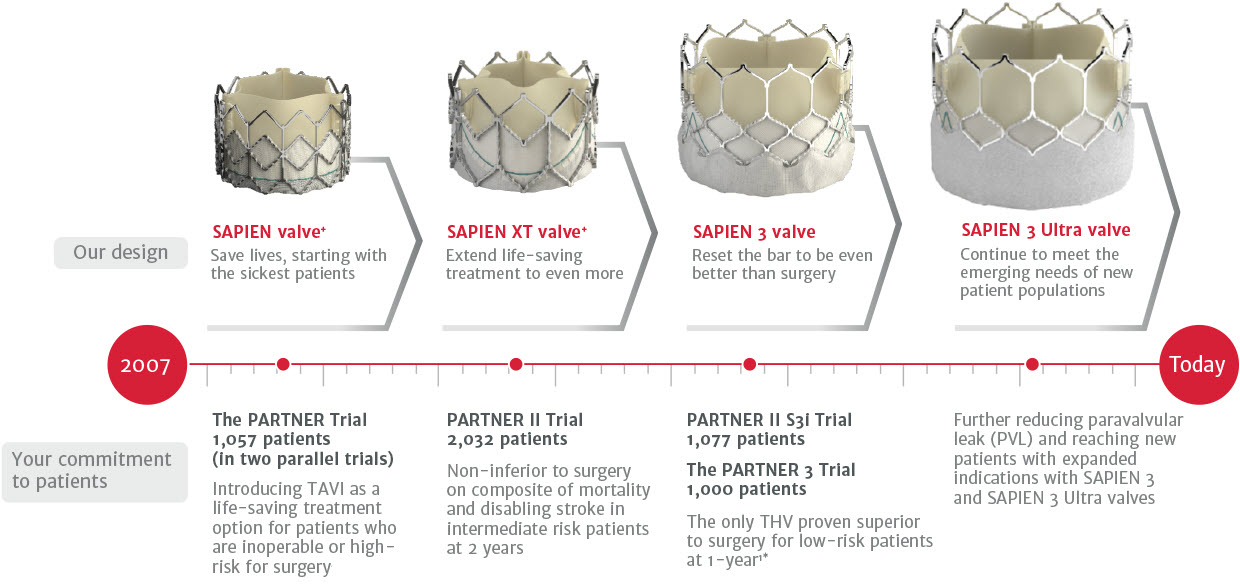
† Data on file, patient clinical trial enrollment data.
*The PARTNER 3 Trial, SAPIEN 3 TAVR proven superior to surgery on the primary endpoint all-cause death, all stroke, and rehospitalization (valve-related or procedure-related and including heart failure) and multiple pre-specified secondary endpoints.
Learn more
Find the latest data, training programs, and resources designed to optimise patient care and increase efficiencies.
References:
1. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-4078 v1.0
