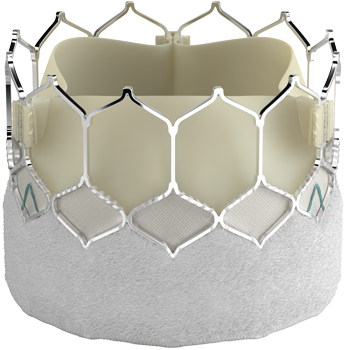
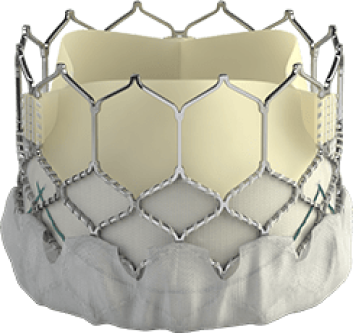
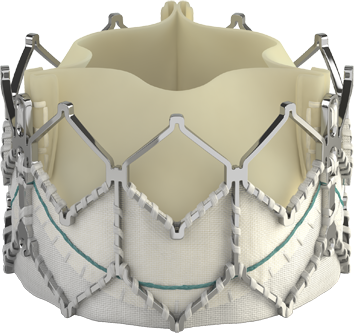
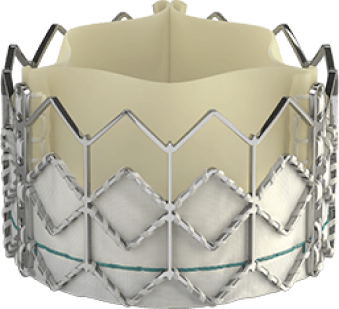
Introducing the Edwards SAPIEN 3 Ultra RESILIA valve
Building on the benefits of the SAPIEN 3 platform3,4,5, the SAPIEN 3 Ultra RESILIA valve features RESILIA tissue, an advanced calcium-blocking technology.1,2 The SAPIEN 3 Ultra RESILIA valve takes TAVI even further with the potential to improve valve longevity and reduce reintervention.1
Learn about SAPIEN 3 Ultra RESILIA valve*No clinical data are available to evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
†RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards Lifesciences in a juvenile sheep model. Flameng W, et al. J Thorac Cardiovasc Surg. 2015;149 (1):340-345.