Edwards SAPIEN 3 Ultra TAVI
Bicuspid Treatment Options
Edwards SAPIEN 3 Ultra TAVI
Bicuspid Treatment Options
Bicuspid aortic valve disease is the most common congenital heart defect in adults1
worldwide population1
develop major complications, including aortic stenosis and regurgitation2
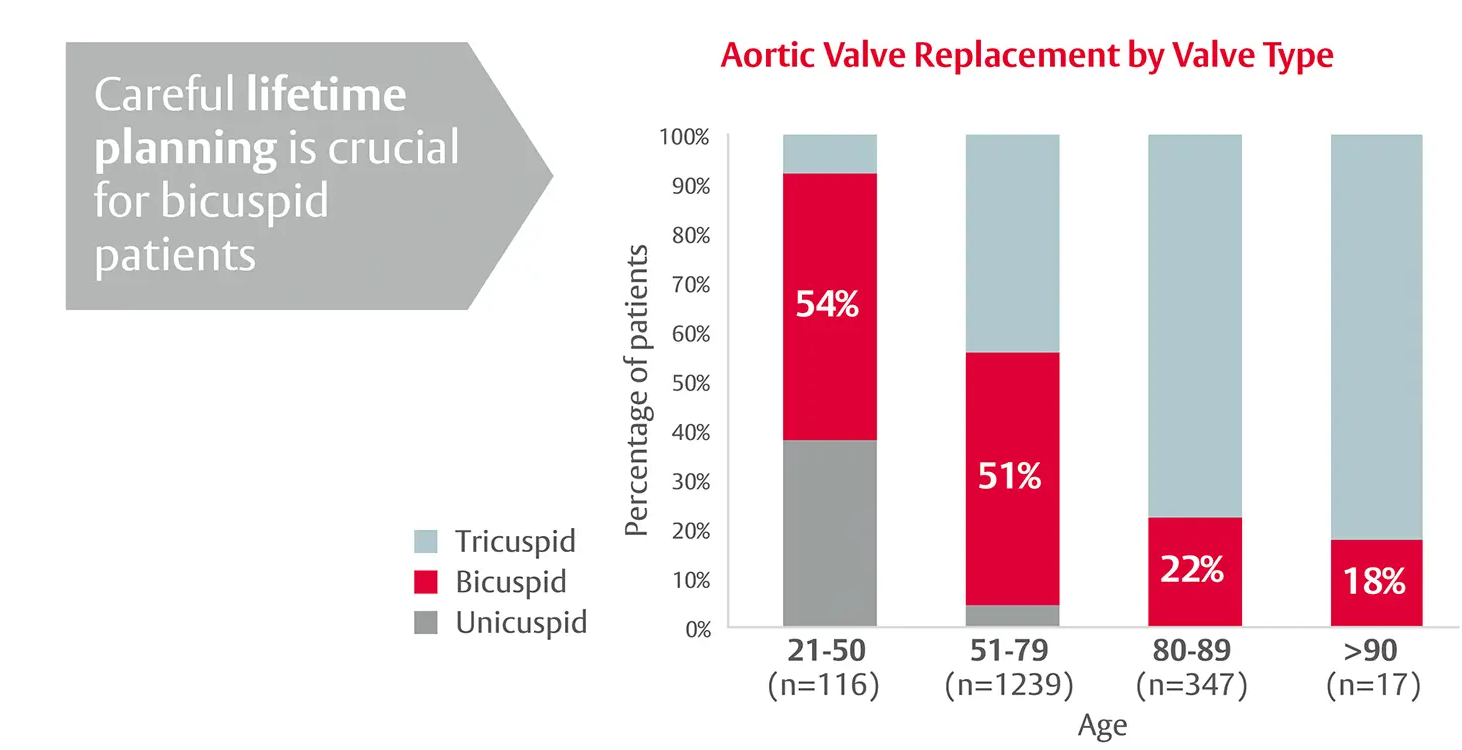
Congenital bicuspid aortic valve disease is more prevalent in younger patients requiring AVR3
1. Sievers HH. et al. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007 May;133(5):1226-33. / 2. Jermihov PN. et al. Effect of Geometry on the Leaflet Stresses in Simulated Models of Congenital Bicuspid Aortic Valves. Cardiovasc Eng Technol. 2011 Mar;2(1):48-56. / 3. Roberts WC. et al. Frequency of congenitally bicuspid aortic valves in patients ≥ 80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol. 2012 Jun 1;109(11):1632-6.
Historically, pivotal randomized trials excluded bicuspid patients due to perceived anatomical challenges for TAVI1
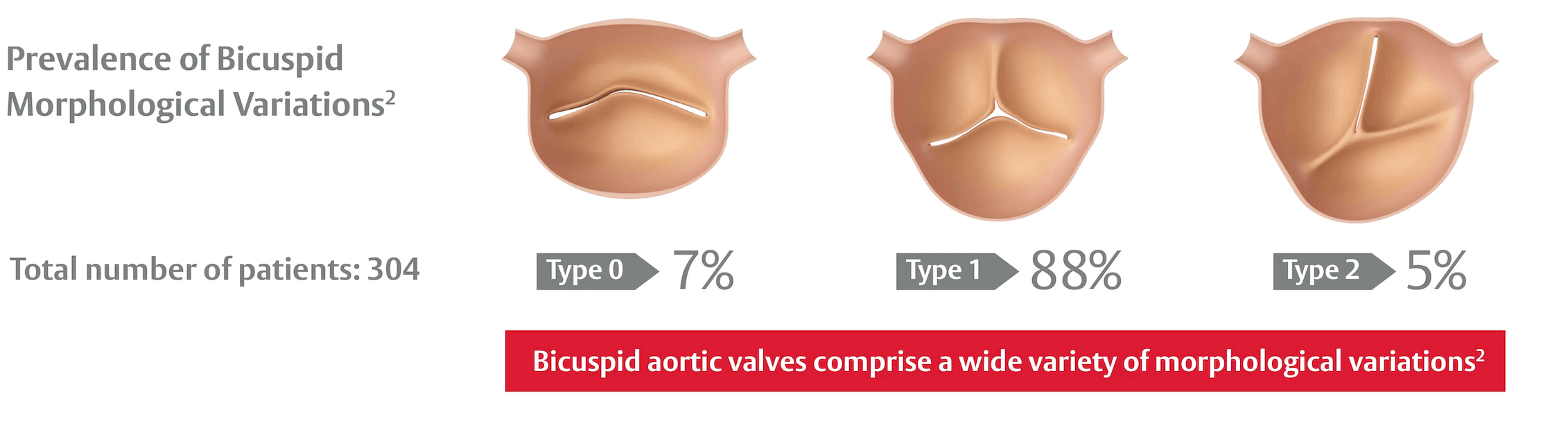
1. Das R. et al. Transcatheter Treatment of Bicuspid Aortic Valve Disease: Imaging and Interventional Considerations. Front Cardiovasc Med. 2018 Jul 19;5:91. / 2. Sievers HH. et al. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007 May;133(5):1226-33.
The Heart Team needs to evaluate several factors that can impact therapy selection1
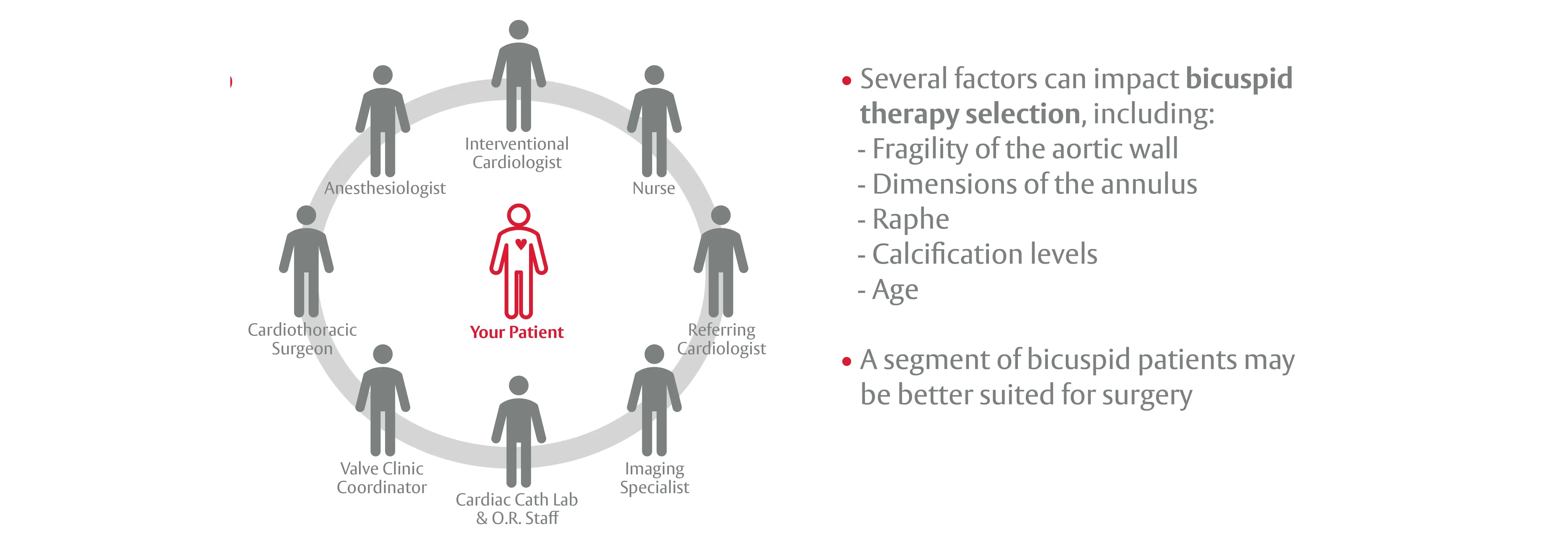
1. Anwaruddin S. et al. Systematic Approach Toward Transcatheter Treatment of BAV Disease: One Size Does Not Fit All. J Am Coll Cardiol. 2020 Sep 1;76(9):1031-1033.Anwaruddin S. et al. Systematic Approach Toward Transcatheter Treatment of BAV Disease: One Size Does Not Fit All. J Am Coll Cardiol. 2020 Sep 1;76(9):1031-1033.
The SAPIEN 3 valve platform has been studied across all surgical risk classes in several international registries
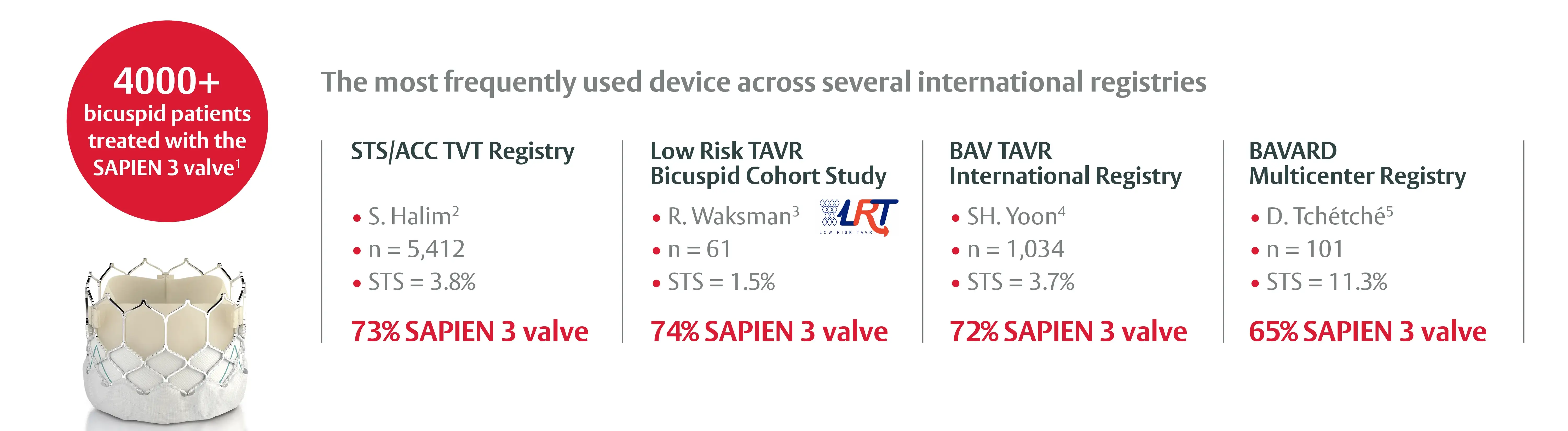
1. STS/ACC TVT Registry, Data on File at Edwards Lifesciences. / 2. Kim SA. et al. Outcomes of Transcatheter Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Disease: A Report From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2020 Mar 31;141(13):1071-1079. / 3. Waksman R. et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Bicuspid Aortic Valve Stenosis. JACC Cardiovasc Interv. 2020 May 11;13(9):1019-1027. / 4. Yoon SH. et al. Bicuspid Aortic Valve Stenosis Transcatheter Aortic Valve Replacement Registry Investigators. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020 Sep 1;76(9):1018-1030. / 5. Tchetche D. et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ Cardiovasc Interv. 2019 Jan;12(1):e007107.
Recent publications have however demonstrated that TAVI can be a safe and effective alternative to surgery1
![Infographic comparing outcomes for bicuspid vs tricuspid patients treated with SAPIEN 3 TAVI. Left gray arrow text: 'In selected bicuspid patients SAPIEN 3 TAVI achieves outcomes similar to tricuspid patients' above a photo of the SAPIEN 3 valve. Right: 'TVT-Registry — Matched cohort' table with columns Bicuspid and Tricuspid: 'Median Age (years) 74 vs 74'; 'Mean STS Score (%) 4.9 vs 5.1'. Kaplan–Meier chart with legend (gray=Bicuspid, red=Tricuspid), y-axis 'All-cause mortality or stroke (%)', x-axis 'Time in months'. Twelve-month rates: Tricuspid 14.1%, Bicuspid 12.9%. Statistics: 'HR: 0.97 [95% CI: 0.81 to 1.16] P=0.74'. Number at risk (months 0, 3, 6, 9, 12): Bicuspid 2691, 1234, 1196, 1135, 910; Tricuspid 2691, 1341, 1296, 1226, 952.](/storage/images/3968/landingpages5.webp)
1. Makkar RR. et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA. 2019 Jun 11;321(22):2193-2202.
The SAPIEN 3 valve platform demonstrates excellent PVL performance in bicuspid patients

1. Makkar R. et al. Outcomes of Transcatheter Aortic Valve Replacement with Balloon Expandable Sapien3 Valve in Bicuspid Aortic Stenosis:; Presented at ACC 2019, New Orleans, USA.
2. Williams M. et al. The PARTNER 3 Bicuspid Registry for SAPIEN 3 TAVR in Low Surgical Risk Patients; Presented at TCT 2020; October 2020, tctconnect.com
At the time of the first implant, several factors need to be considered
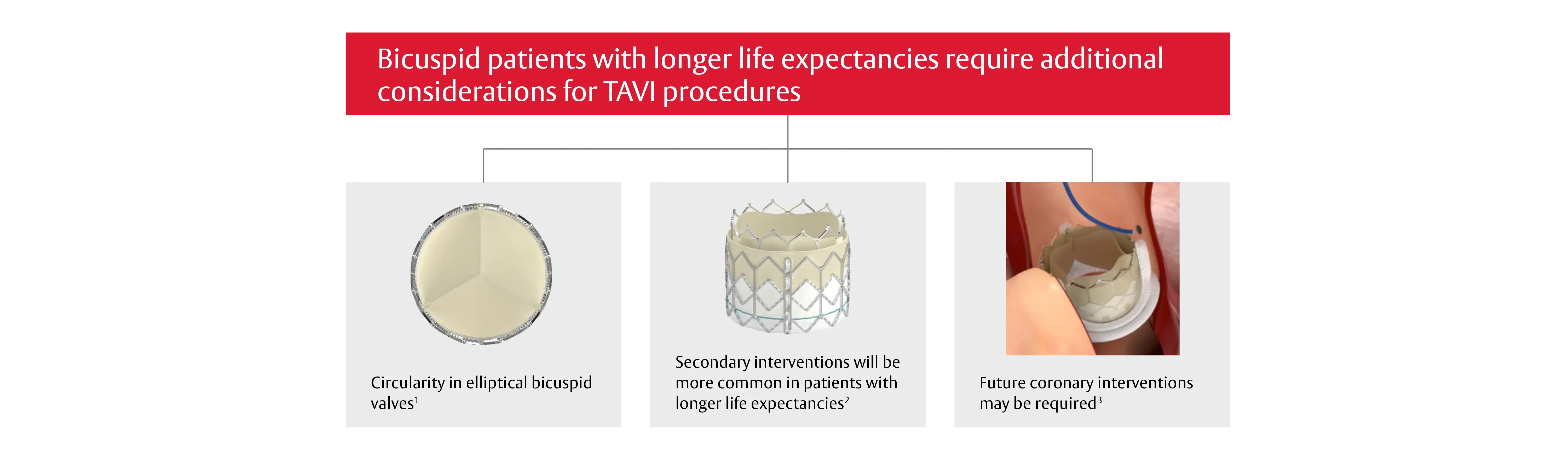
1. Tchetche D. et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ Cardiovasc Interv. 2019 Jan;12(1):e007107. / 2. Pasala TKR. et al. Transcatheter Aortic Valve Replacement for All-comers With Severe Aortic Stenosis: Could It Become a Reality?. Rev Esp Cardiol (Engl Ed). 2018;71(3):141-145. / 3. Yudi MB, et al. Coronary Angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. JACC Vol 71, No 12, 2018.
Circularity is the hallmark of the SAPIEN 3 valve design
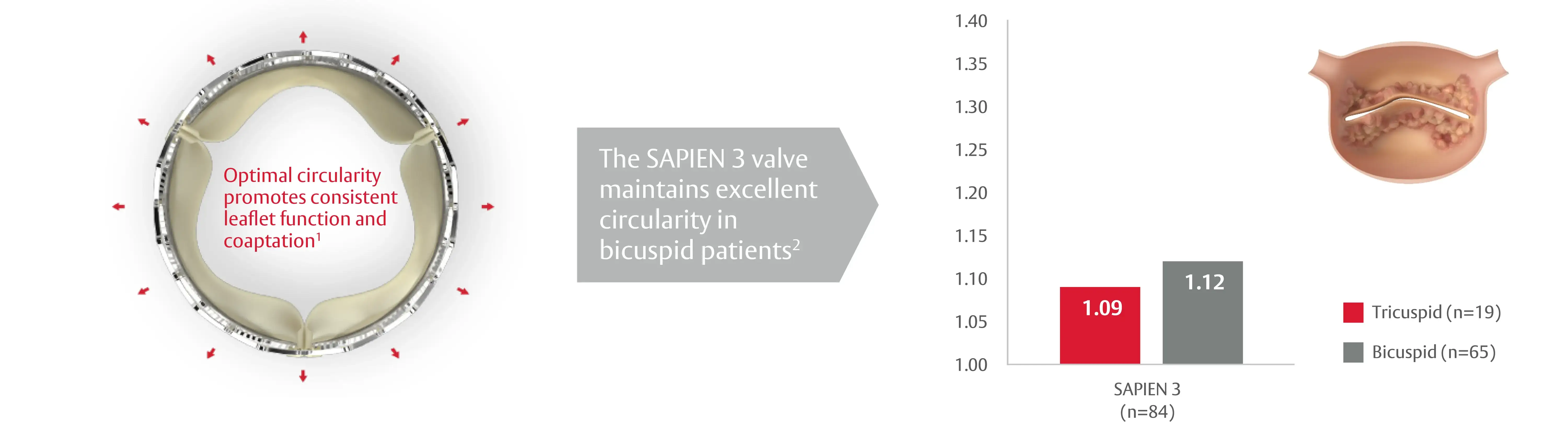
1. Binder RK et al. Transcatheter aortic valve replacement with the SAPIEN 3: a new balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv. 2013 Mar;6(3):293-300.
2. Tchetche D. et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ Cardiovasc Interv. 2019 Jan;12(1):e007107.
The need for post-TAVI coronary access is expected to increase in patients with longer life expectancies
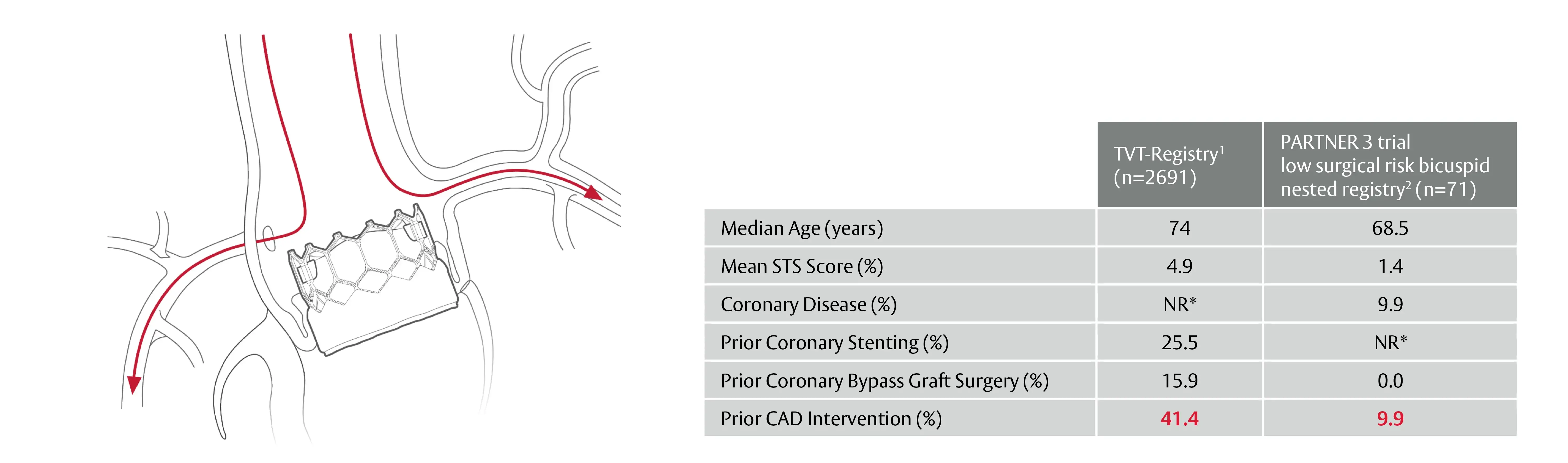
* Not Reported / 1. Makkar RR. et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA. 2019 Jun 11;321(22):2193-2202. / 2. Williams M. et al. The PARTNER 3 Bicuspid Registry for SAPIEN 3 TAVR in Low Surgical Risk Patients; Presented at TCT 2020; October 2020, tctconnect.com / 3. Image adapted from Sondergaard L., De Backer O. (2018). Transcatheter aortic valve implantation: don’t forget the coronaries! EuroInterv, 14(2), 147-9.
Welcome to the Higher Standard Your Standard.
Find out moreTAVI in Bicuspid Aortic Valve patients: SAPIEN 3 platform, a safe and efficient option
Speakers: R. Parma, J. Kempfert, D. Blackman, B. Garcia del Blanco
TAVI in Bicuspid Aortic Valve patients. SAPIEN 3 platform, a safe and efficient option
Moderated by Philippe Généreux. Speakers: J. Kempfert, R. Parma, D. Tchétché
TAVI in Bicuspid Aortic Valves
Speakers: D. Blackman, S. Doshi
SAPIEN 3 Ultra in in Bicuspid Aortic Valve Type 0
Deutsches Herzzentrum – Berlin
Performed by: J. Kempfert, C. Klein
Introduced by: A. Unbehaun
SAPIEN 3 Ultra in Bicuspid Aortic Valve Type 1
Deutsches Herzzentrum – Berlin
Performed by: J. Kempfert, C. Klein
Introduced by: A. Unbehaun
Bicuspid aortic valve case presentation – part 1
Presented by: Dr. Didier Tchetche, Dr. Nicolas Dumonteil
Bicuspid aortic valve case presentation – part 2
Presented by: Dr. Didier Tchetche, Dr. Nicolas Dumonteiln
Performed by: J. Kempfert, C. Klein
Introduced by: A. Unbehaun
Interview with J. Kempfert
Deutsches Herzzentrum – Berlin
As a subscriber you will benefit from:
Publications
 Featuring the latest cardiology news, clinical trials and real-world evidence in aortic stenosis (AS) management.
Featuring the latest cardiology news, clinical trials and real-world evidence in aortic stenosis (AS) management.
Panel discussions
 Involving leading cardiologists debating the latest topics in transcatheter aortic valve implantation (TAVI) and AS management.
Involving leading cardiologists debating the latest topics in transcatheter aortic valve implantation (TAVI) and AS management.
Conference headlines
 Providing insights into the major developments from the latest cardiology events.
Providing insights into the major developments from the latest cardiology events.
Guidelines updates
 Highlighting the areas of change within any updated aortic stenosis treatment guidelines.
Highlighting the areas of change within any updated aortic stenosis treatment guidelines.
Thank You
Thank you for signing up to the heartvalves.com newsletter. You will now receive emails on the latest developments and industry insights in heart valve innovation and technology.
Back to homeNo clinical data are available to evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-10024 v1.0