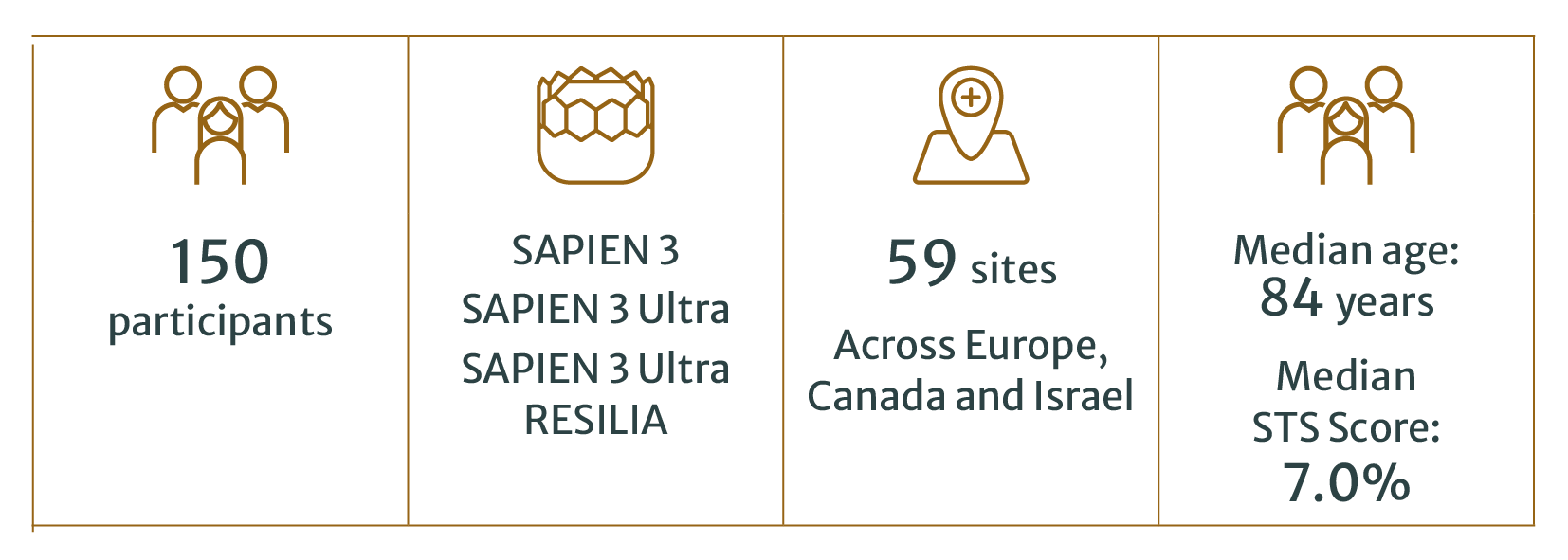
First prospective, multi-center observational study evaluating clinical outcomes of THV-in-THV procedures with the SAPIEN 3 platform. The aim is to assess short- and long-term data on patients treated with the SAPIEN 3 platform for failure of a previously implanted THV.
ReTAVI Registry
Patients undergoing a THV-in-THV
Procedure with SAPIEN Platform
ReTAVI Registry
Patients undergoing a THV-in-THV
Procedure with SAPIEN Platform
The ReTAVI Registry: Investigator-initiated study
First Prospective, Multicenter, International registry with patients treated with the SAPIEN 3 platform in a degenerated THV.
G. Tarantini, R. Parma - Evaluation of Clinical Outcomes of Patients Undergoing a Redo-TAVI Procedure; a Multicenter Prospective Observational Registry. Presented at TCT 25th of October 2025, San Francisco, USA.

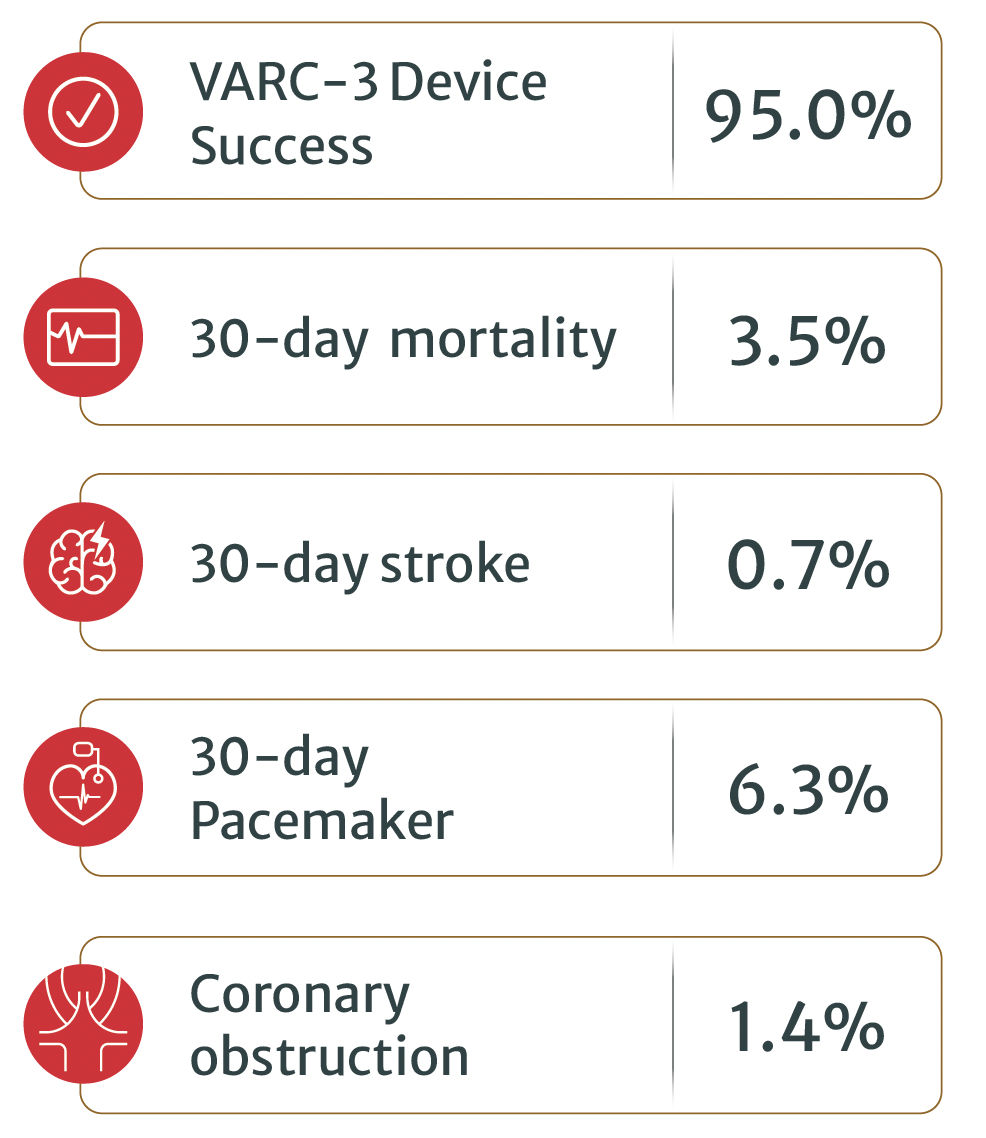
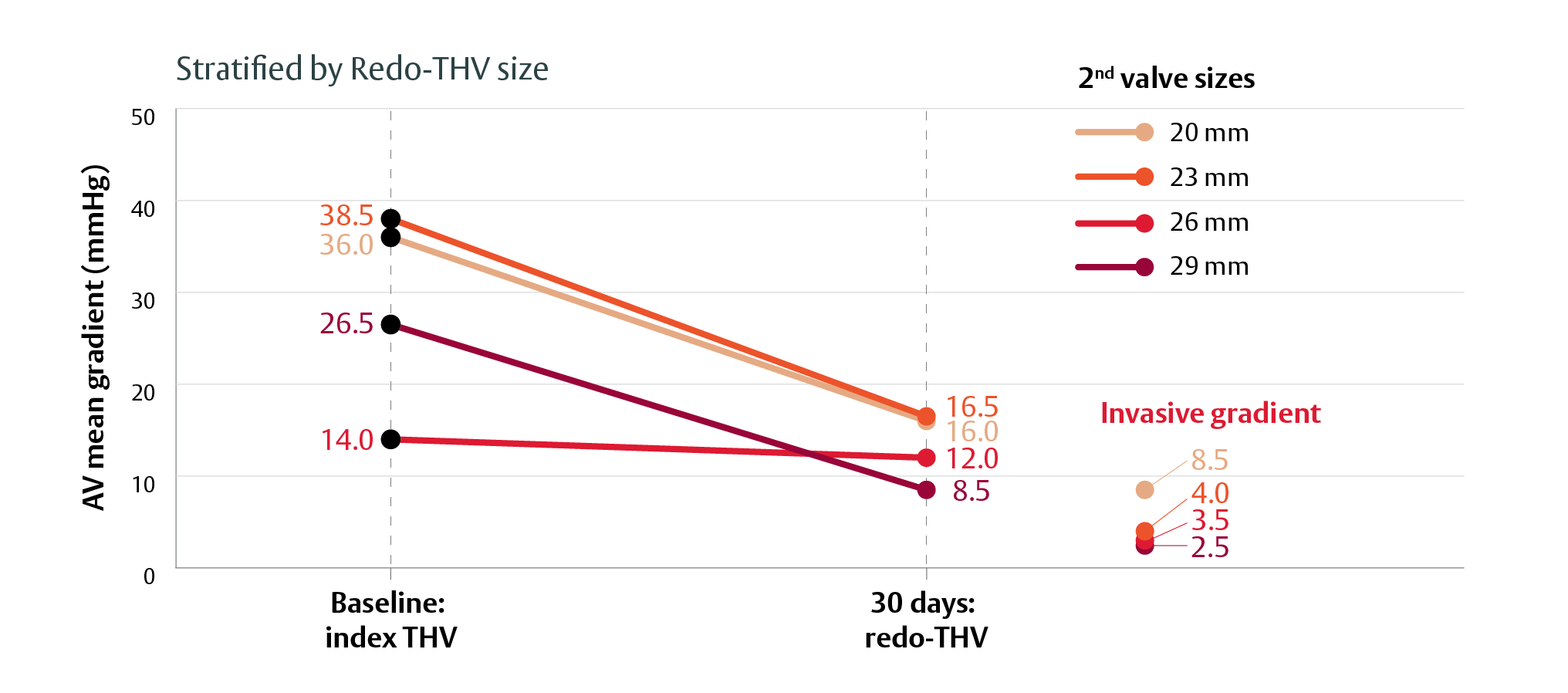
THV-in-THV with the SAPIEN 3 platform provides excellent clinical outcomes at discharge and 30 days1
THV-in-THV using the SAPIEN platform is a safe and effective reintervention strategy for aortic THV failure
ReTAVI registry - Generating impactful evidence for THV-in-THV procedures with the SAPIEN 3 platform
Between 1.4% and 2.8% of all patients undergoing TAVI require a second THV2,3,4. With the broadening of TAVI indications and treatment of patients with a longer life expectancy, these procedures are expected to grow in the coming years5. The first dedicated prospective study is an important step to better understand outcomes and best practices.
Main clinical outcomes (n=143):
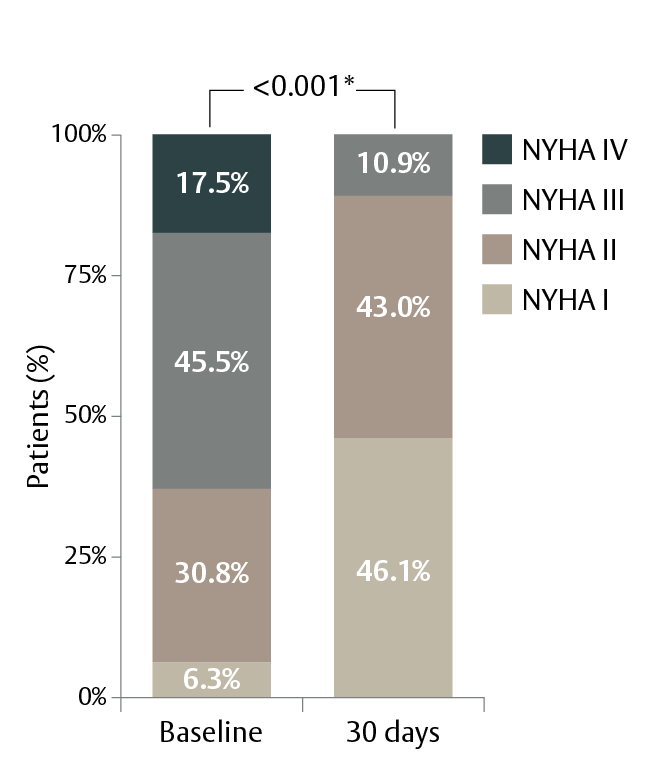
Patients experienced significant improvement in their quality of life
NYHA Class scores at 30 days (n=143):
98.6% of no coronary obstruction with the SAPIEN 3 platform in the studied index valve1
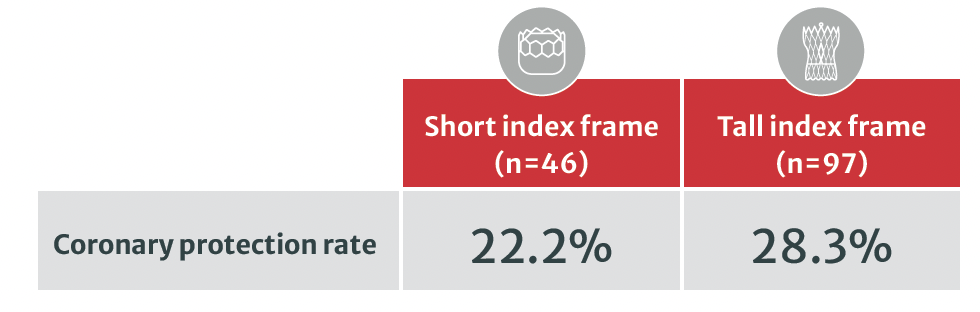
Coronary protection was more used in tall frame valves
Consistent hemodynamic improvement across anatomies, index valve types and sizes with the SAPIEN 3 platform1
The ReTAVI Registry results were recently presented at TCT 2025
Dr. Giuseppe Tarantini
Presented at TCT, 25th of October 2025, San Francisco, USA
Download the clinical summary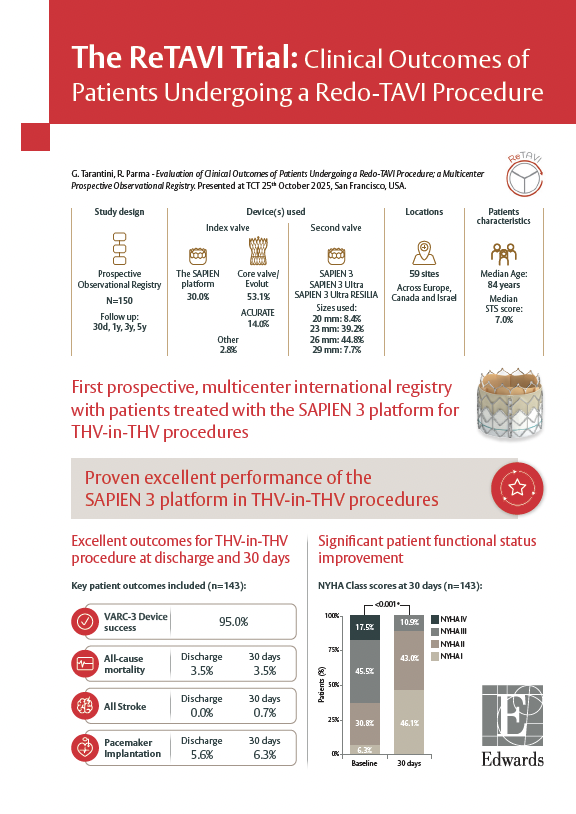
Stay informed with our newsletter
Thank you!
Thank you for signing up to the heartvalves.com newsletter. You will now receive emails on the latest developments and industry insights on heart valve innovation and technology.
References:
1. G. Tarantini - Early Outcomes of Redo-TAVI with SAPIEN Platform in a Real-World Prospective Cohort. Presented at TCT 25th of October,
San Francisco, USA
2. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N
Engl J Med. 2010; 363(17): 1597-1607.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J
Med. 2011; 364(23): 2187-2198
4. Toggweiler S, Wood DA, Rodés-Cabau J, et al. Transcatheter valve-in-valve implantation for failed balloon-expandable transcatheter
aortic valves. JACC Cardiovasc Interv. 2012; 5(5): 571-577.
5. Adamo, M., Blackman, D., Parma, R., Khokhar, A. (Discussants), & Abdel-Wahab, M. (Anchorperson). (2025, May 22). Redo TAVI – How to
treat different TAVI platforms when they fail. Session presented at EuroPCR 2025, Paris, France
6. Beaver T, Bavaria JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis.
J Thorac Cardiovasc Surg. 2024 Sep;168(3):781-791.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Clinical data on surgical valves with RESILIA tissue up to 7-year follow-up have been published, with additional follow-up to 10-years in progress(6).
PP--EU-11336 v1.0